Hydrogen (H2)
Hydrogen atoms only have 1 electron and they only need 1 more to compete their shell (remember: the first shell only needs two electrons to be complete). To complete their shell, they form a covalent bond. Only one pair of electrons is shared between them, this molecule is known as H2 (hydrogen gas)
Chlorine (Cl2)
Much like hydrogen, chlorine atoms also only need one more electron...
This is Cl2 (as there are 2 Cl atoms in the molecule)
Hydrogen chloride (HCl)
As hydrogen and chlorine both only need one electron to complete their outer shell, they can bond with each other, this forms HCl...
Water (H2O)
Oxygen atoms have 6 electrons on their outer shell and therefore need 2 more electrons to complete their shell. However, hydrogen only needs one. This means that one oxygen atom must bond with two hydrogen atoms...
Methane (CH4)
Carbon has 4 outer electrons, therefore it needs 4 more to complete its outer electron shell. However, hydrogen only needs 1 more electron. This means that one carbon atom bonds with four hydrogen atoms
Ammonia (NH3)
Nitrogen has 5 electrons on its outer shell, so needs 3 more electrons to compete its shell, but hydrogen only needs 1 more electron. This means that 1 nitrogen atom bonds with 3 hydrogen atoms...
Oxygen (O2)
In oxygen gas one oxygen atom shares TWO pairs of electrons with another oxygen atom to complete its shell, this is known as a double covalent bond
Nitrogen (N2)
Nitrogen atoms have 5 electrons on their outer shell, therefore they need 3 more electrons to complete their shell. This means that two nitrogen atoms share THREE pairs of electrons to fill their outer shells, this is known as a triple covalent bond

Carbon dioxide (CO2)
In carbon dioxide. two oxygen atoms share two pairs of electrons with a carbon atom, this forms two double covalent bonds
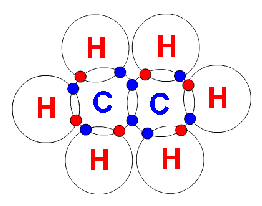
Ethane (C2H6)
In ethane there are two carbon atoms and six hydrogen atoms. Each of the six hydrogen atoms share their only electron with one of the two carbon atoms (each carbon atom bonds with 3 hydrogen atoms), the two carbon atoms then share their last electron with each other.
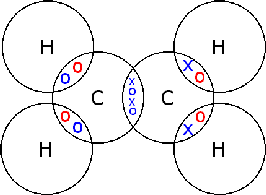
Ethene (C2H4)
In there there are two carbon atoms and four hydrogen atoms. The four hydrogen atoms each share their only electron with one of two carbon atoms, the two carbon atoms then share their lat two electrons with each other, forming a carbon-carbon double covalent bond.








No comments:
Post a Comment