Diamond
In diamond, each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. As a result, diamond is very hard and has a high melting point. This explains why it is used in cutting tools
Graphite
In graphite, each carbon atom is joined to only three other carbon atoms, this results in carbon sheets that are 'stacked' on top of each other. These layers can slide over each other, this means that graphite is much softer than diamond. It is used in pencils, and as a lubricant
NOTE: Diamond does not conduct electricity but graphite does
A blog covering and explaining the Edexcel IGCSE Chemistry specification for the 2016 summer exams. If you are doing just double science, you do not need to learn the stuff for paper two, if you are doing triple you will need to learn all (GOOD LUCK!) I have separated the papers to make files easier to find. Hope it helps :)
Showing posts with label covalent substances. Show all posts
Showing posts with label covalent substances. Show all posts
Tuesday, 22 March 2016
1.43 explain the high melting and boiling points of substances with giant covalent structures in terms of the breaking of many strong covalent bonds
Giant covalent structures are very like giant ionic structures only they do not have charged ions. Instead, all atoms are bonded to each other by strong covalent bonds. There are a lot of these bonds which means it takes a lot of energy to break them, therefore they have very high melting and boiling points
1.42 explain why substances with simple molecular structure have low melting and boiling points in terms of the relatively weak forces between the molecules
The atoms within a molecule are held together with strong covalent bonds. In contrast, the intermolecular forces between molecules are very weak and as a result the boiling and melting points of simple molecular substances are very low (because the molecules are easily parted from each other)
1.41 understand that substances with simple molecular structures are gases or liquids, or solids wit low melting points
You can tell when a substance has a simple molecular structure from its physical state (at room temperature). Most molecular substances are liquid or gas (at room temperature) but some are solid with a low melting point
1.40 explain, using dot and cross diagrams, the formation of covalent compounds by electron sharing for the following substances...
For each of the diagrams I have added a little explanation of what's happening to aid understanding, if you know what happens ignore the writing, otherwise read it as it may be useful :)
Hydrogen (H2)
Hydrogen atoms only have 1 electron and they only need 1 more to compete their shell (remember: the first shell only needs two electrons to be complete). To complete their shell, they form a covalent bond. Only one pair of electrons is shared between them, this molecule is known as H2 (hydrogen gas)

Chlorine (Cl2)
Much like hydrogen, chlorine atoms also only need one more electron...

This is Cl2 (as there are 2 Cl atoms in the molecule)
Hydrogen chloride (HCl)
As hydrogen and chlorine both only need one electron to complete their outer shell, they can bond with each other, this forms HCl...

Water (H2O)
Oxygen atoms have 6 electrons on their outer shell and therefore need 2 more electrons to complete their shell. However, hydrogen only needs one. This means that one oxygen atom must bond with two hydrogen atoms...

Methane (CH4)
Carbon has 4 outer electrons, therefore it needs 4 more to complete its outer electron shell. However, hydrogen only needs 1 more electron. This means that one carbon atom bonds with four hydrogen atoms

Ammonia (NH3)
Nitrogen has 5 electrons on its outer shell, so needs 3 more electrons to compete its shell, but hydrogen only needs 1 more electron. This means that 1 nitrogen atom bonds with 3 hydrogen atoms...

Oxygen (O2)
In oxygen gas one oxygen atom shares TWO pairs of electrons with another oxygen atom to complete its shell, this is known as a double covalent bond

Nitrogen (N2)
Nitrogen atoms have 5 electrons on their outer shell, therefore they need 3 more electrons to complete their shell. This means that two nitrogen atoms share THREE pairs of electrons to fill their outer shells, this is known as a triple covalent bond

Carbon dioxide (CO2)
In carbon dioxide. two oxygen atoms share two pairs of electrons with a carbon atom, this forms two double covalent bonds
Ethane (C2H6)
In ethane there are two carbon atoms and six hydrogen atoms. Each of the six hydrogen atoms share their only electron with one of the two carbon atoms (each carbon atom bonds with 3 hydrogen atoms), the two carbon atoms then share their last electron with each other.
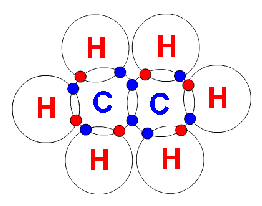
Ethene (C2H4)
In there there are two carbon atoms and four hydrogen atoms. The four hydrogen atoms each share their only electron with one of two carbon atoms, the two carbon atoms then share their lat two electrons with each other, forming a carbon-carbon double covalent bond.
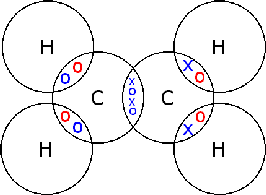
Hydrogen (H2)
Hydrogen atoms only have 1 electron and they only need 1 more to compete their shell (remember: the first shell only needs two electrons to be complete). To complete their shell, they form a covalent bond. Only one pair of electrons is shared between them, this molecule is known as H2 (hydrogen gas)

Chlorine (Cl2)
Much like hydrogen, chlorine atoms also only need one more electron...

This is Cl2 (as there are 2 Cl atoms in the molecule)
Hydrogen chloride (HCl)
As hydrogen and chlorine both only need one electron to complete their outer shell, they can bond with each other, this forms HCl...

Water (H2O)
Oxygen atoms have 6 electrons on their outer shell and therefore need 2 more electrons to complete their shell. However, hydrogen only needs one. This means that one oxygen atom must bond with two hydrogen atoms...

Methane (CH4)
Carbon has 4 outer electrons, therefore it needs 4 more to complete its outer electron shell. However, hydrogen only needs 1 more electron. This means that one carbon atom bonds with four hydrogen atoms

Ammonia (NH3)
Nitrogen has 5 electrons on its outer shell, so needs 3 more electrons to compete its shell, but hydrogen only needs 1 more electron. This means that 1 nitrogen atom bonds with 3 hydrogen atoms...

Oxygen (O2)
In oxygen gas one oxygen atom shares TWO pairs of electrons with another oxygen atom to complete its shell, this is known as a double covalent bond

Nitrogen (N2)
Nitrogen atoms have 5 electrons on their outer shell, therefore they need 3 more electrons to complete their shell. This means that two nitrogen atoms share THREE pairs of electrons to fill their outer shells, this is known as a triple covalent bond

Carbon dioxide (CO2)
In carbon dioxide. two oxygen atoms share two pairs of electrons with a carbon atom, this forms two double covalent bonds
Ethane (C2H6)
In ethane there are two carbon atoms and six hydrogen atoms. Each of the six hydrogen atoms share their only electron with one of the two carbon atoms (each carbon atom bonds with 3 hydrogen atoms), the two carbon atoms then share their last electron with each other.

Ethene (C2H4)
In there there are two carbon atoms and four hydrogen atoms. The four hydrogen atoms each share their only electron with one of two carbon atoms, the two carbon atoms then share their lat two electrons with each other, forming a carbon-carbon double covalent bond.

1.39 understand covalent bonding as a strong attraction between the bonding pair of electrons and the nuclei of the atoms involved in the bond
sorry not much to say here... you just need to learn that in covalent bonding there is a strong attraction between the shared electrons (the 'bonding pair') and the nuclei of the atoms involved
1.38 describe the formation of a covalent bond by sharing a pair of electrons between the two atoms
Covalent bonding is where atoms share electrons with each other so that they have a full outer shell (as aposed to ionic bonding where an electron is 'given away' to another atom)
Subscribe to:
Posts (Atom)


