Halide ions
To test for halide ions, add dilute nitric acid (HNO3) followed by silver nitrate solution (AgNO3). A precipitate will be produces, the colour of this precipitate will determine what ions are present.
Cl - > white precipitate
Br- > cream precipitate
I- > yellow precipitate
NOTE: The silver nitrate solution determines which halide ions are present. The dilute nitric acid is added to 'get rid' of an carbonate or sulphite ions (as these would react with the silver nitrate).
Sulphate ions
To test for sulphate ions (SO42- ), add dilute HCl and then barium chloride solution (BaCl2). If sulphate ions are present, a note precipitate would form (this precipitate is barium sulphate)
NOTE: The HCl is used to 'get rid' of any carbonate ions, and these may impede results if present as they would also produce a precipitate).
Carbonate ions
To test for carbonate ions (CO32- ), add dilute HCl to the sample you are testing. If a gas is produced, collect it and bubble it through limewater. If carbonate ions are present, the limewater will go cloudy as carbon dioxide will be released.
A blog covering and explaining the Edexcel IGCSE Chemistry specification for the 2016 summer exams. If you are doing just double science, you do not need to learn the stuff for paper two, if you are doing triple you will need to learn all (GOOD LUCK!) I have separated the papers to make files easier to find. Hope it helps :)
Wednesday 18 May 2016
1.55 write ionic half equations representing the reactions at the electrodes during electrolysis
Half equations just show what happened at each electrode. Like any equation, they need to be balanced. here are some examples...
2Br- > Br2 + 2e-
Above is an equation of a reaction at an anode. We can tell this because the Br2 loses an electron. This can be inferred as on the left it is 2Br- and on the right it is Br2 (meaning it has lost an electrode as it no longer is a negative ion, it is an atom). As it is 2Br-, 2 electrons must be lost.
2H+ + 2e- > H2
Above is an equation of a reaction at a cathode as 2H+ gains 2 electrons to become H2. The charges cancel out and it is neutral on both sides. As there are 2 'H's, there must be 2 electrons gained.
2Br- > Br2 + 2e-
Above is an equation of a reaction at an anode. We can tell this because the Br2 loses an electron. This can be inferred as on the left it is 2Br- and on the right it is Br2 (meaning it has lost an electrode as it no longer is a negative ion, it is an atom). As it is 2Br-, 2 electrons must be lost.
2H+ + 2e- > H2
Above is an equation of a reaction at a cathode as 2H+ gains 2 electrons to become H2. The charges cancel out and it is neutral on both sides. As there are 2 'H's, there must be 2 electrons gained.
1.46 understand that a metal can be described as a giant structuree of positive ions surrounded by a sea of delocalised electrons
In a metal, the atoms are ionically bonded in a giant 3-D structure. The outer shell electrons become detached (making positive ions, cations). The cations are 'floating' in a sea of delocalised electrons. The metal structure stays together because the cations (+) are attracted to the electrons (-). This is known as a metallic bond.
1.31 deduce the charge of an ion from the electronic configuration of the atom from which the ion is formed
An atom with less then four electrons on its outer shell will want to lose electrons because that is the quickest way for it to have a full outer shell. So we know the atom will lose electrons (this makes positive ions).
Atoms with more than four electrons will gain electrons to fill their outer shell (as it is easier than losing electrons). This will result in negative ions.
Here are some examples...
Na has the electronic configuration of 2.8.1 (it has 1 electron on it's outer shell, so it is easiest to lose 1 electron to make a full outer shell). This results in the positive ion (a cation), Na+, which has the electronic configuration of 2.8
Cl has the electronic configuration of 2.8.7 (it has 7 electrons on it's outer shell, so it is easiest to gain 1 electron to make a full outer shell). This results in the negative ion (an anion), Cl-, which has the electronic configuration of 2.8.8
Cl has the electronic configuration of 2.8.7 (it has 7 electrons on it's outer shell, so it is easiest to gain 1 electron to make a full outer shell). This results in the negative ion (an anion), Cl-, which has the electronic configuration of 2.8.8
Tuesday 17 May 2016
5.22 understand that nitrogen from air, and ydrogen from natural gas or the cracking of hydrocarbons, are used in the manufacture of ammonia
Ammonia is manufactured using hydrogen and nitrogen (as ammonia is NH3). The equation for the reaction is...
N2 (g) + 3H2 (g) ⇌ + 2NH3 (g) (+heat)
Hydrogen is sourced from natural gas. Nitrogen is sourced from the air (as the air is 78% nitrogen).
The manufacture of ammonia with hydrogen and nitrogen is reversible, therefore, as soon as ammonia is made, it starts to turn back into nitrogen and hydrogen. This mean that not all of the nitrogen and hydrogen is converted to ammonia and not all ammonia is converted into nitrogen and hydrogen (because, as soon as nitrogen and hydrogen are produced, it starts to turn into ammonia). This means the reaction reached a dynamic equilibrium.
N2 (g) + 3H2 (g) ⇌ + 2NH3 (g) (+heat)
Hydrogen is sourced from natural gas. Nitrogen is sourced from the air (as the air is 78% nitrogen).
The manufacture of ammonia with hydrogen and nitrogen is reversible, therefore, as soon as ammonia is made, it starts to turn back into nitrogen and hydrogen. This mean that not all of the nitrogen and hydrogen is converted to ammonia and not all ammonia is converted into nitrogen and hydrogen (because, as soon as nitrogen and hydrogen are produced, it starts to turn into ammonia). This means the reaction reached a dynamic equilibrium.
Sunday 15 May 2016
5.21 understamd that condensation polymerisation produces a small molecule, such as water, as well as the polymer
When condensation polymerisation occurs, a small molecule (such as water) is produces, as well as the monomer.
5.20 understand that some polymers, such as nylon, form by a different process called condensation poymerisation
Most often, condensation polymerisation involves two different types of monomer. These monomers react together forming bonds between them, making polymer chains. However, for each new bond that forms, a molecule (e.g. water) is lost.
5.19 explain that addition polymers are hard to dispose of as their inertness means that they do not easily biodegrade
Most addition polymers are inert. All this means is that they do not react easily (due to the fact that the carbon bonds are very strong and hard to break). This means that it takes a really long time for addition polymers to biodegrade. Furthermore, burning plastics releases toxic chemicals so that's not a good idea either. Because of this, it is hard to dispose of polymers (that's why we recycle them).
5.18 describe some uses for polymers, including poly(ethene), poly(propene) and poly(chloroethene)
Polyethene is light and stretchy making it ideal for packaging such as plastic bags, water bottles/food containers and carpets.
Polypropene is a tough polymer but is quite flexible and heat resistant too, this makes it ideal for making things like crates
Polychloroethene is used to make clothes, drainpipes and insulating cables
Polypropene is a tough polymer but is quite flexible and heat resistant too, this makes it ideal for making things like crates
Polychloroethene is used to make clothes, drainpipes and insulating cables
5.17 deduce the structure of a monomer from the repeat unit of an addition polymer
To find the structure of a monomer, take the repeat unit of an addition polymer and add a double bond between the two carbons.
5.16 draw the repeat unit of addition polymers, including poly(ethene), poly(propene) and poly(chloroethene)
5.15 understand that an addition polymer is formed by joining up many small molecules called monomers
A polymer is just a very long saturated chain (saturated as it has no carbon double bonds). Small molecules called monomers join together to make polymers. One type of polymer is an addition polymer. The monomers that make up addition polymers are alkenes as they have a carbon-carbon double bond. If alkenes are put under a high pressure with a catalyst, they will break their carbon-carbon double bond and polymerise (join together), forming a polymer.
5.14 describe how lng-chain alkanes are converted to alkenes and shorter-chaiin alkanes by catalytic cracking, using silica or alumina as the atalyst and a temperature in the range of 600-700°C
If carrying out the reaction in a lab...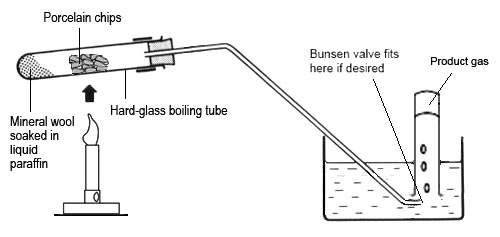
1- Heat the hydrocarbon (e.g. paraffin).
2- After a few seconds, move the Bunsen burner to heat the silica/aluminium catalyst
3- Alternate between the two until the paraffin vaporises and the catalyst glows red
4- The heated paraffin vapour cracks as it passes over the heated catalyst
5- small alkanes collect at the end of the boiling tube, while alkene gases travel down the delivery tube
6- The alkenes are collected through water using a glass jar
In industry, vapourised hydrocarbons are passed over a powdered catalyst at about 600-700°C. silica and aluminium are used as catalysts.
Notes credit: CGP

1- Heat the hydrocarbon (e.g. paraffin).
2- After a few seconds, move the Bunsen burner to heat the silica/aluminium catalyst
3- Alternate between the two until the paraffin vaporises and the catalyst glows red
4- The heated paraffin vapour cracks as it passes over the heated catalyst
5- small alkanes collect at the end of the boiling tube, while alkene gases travel down the delivery tube
6- The alkenes are collected through water using a glass jar
In industry, vapourised hydrocarbons are passed over a powdered catalyst at about 600-700°C. silica and aluminium are used as catalysts.
Notes credit: CGP
5.13 understand that fractional distillation of crude oil produces more long-chain hydrocarbons than can be used directly and fewer short-chain hydrocarbons than required and explain why this makes cracking necessary
Fractional distillation produces lots of long chain hydrocarbons and not many short chain hydrocarbons in relative. The short chain hydrocarbons are useful but long chain hydrocarbons are not. This means we have to break the long chain hydrocarbons into short chain hydrocarbons as demand for short chain hydrocarbons is high. This is done in a process known as cracking. This process is a form of thermal decomposition, which just means breaking down the molecules into simpler molecules by heating them.
5.12 understand that nitrogen oxides and sulfur dioxide are pollutant gases which contribute to acid raid, and describe the problems caused by acid rain
Sulfur dioxide mix with clouds forming dilute sulfuric acid, which is very acidic. Nitrous oxide also mixes with clouds, forming nitric acid. These fall as acid rain which causes lakes to become acid in (killing plants and animals), kills trees and damages limestone buildings and statues.
There have also been links made between acid rain and human health although these are not proven.
There have also been links made between acid rain and human health although these are not proven.
Wednesday 4 May 2016
5.11 understand that, in car engines, the temperature reached is high enough to allow nitrogen and oxygen from air to react, forming nitrogen oxides
In car engines, crude oil fractions (such as diesel or petrol) are burnt (as fuels). When fossil fuels are burnt, nitrogen oxide and sulfur dioxide are always released, always. When the temperature is high enough, the nitrogen and oxygen in the air react (this creates nitrous oxides, such as nitrogen monoxide and nitrogen dioxide).
NOTE: remember nitrogen oxide and sulfur dioxide are always produced when fossil fuels are burnt.
NOTE: remember nitrogen oxide and sulfur dioxide are always produced when fossil fuels are burnt.
5.10 understand that incomplete combustion of fuels may produce carbon monoxide and explain that carbon monoxide is poisonous because it reduces the capacity of the blood from air to react, forming nitrogen oxides
First of all, incomplete combustion just means that the substance/thing was burnt without lots of oxygen present. When hydrocarbon fuels (for example gas or petrol) is burnt without oxygen, carbon monoxide is produced (as apposed to carbon dioxide, with complete combustion). This is bad because carbon monoxide is poisonous. This is because it combines with haemoglobin (this carried oxygen in your blood), preventing as much oxygen to get to your cells (as less oxygen can bind with the haemoglobin as there is lots of carbon monoxide combined with the haemoglobin). Therefore, less oxygen is being carried around your body (in your blood), this can lead to fainting, a coma or even death (in severe circumstances) as your cells are effectively being starved of oxygen.
Sorry for delays!
As I have been back at school I have not been able to keep up with the blog as much as I was over Easter break.. I leave school this Friday for study leave and will be finishing all blogs next week (iGCSE Chemistry, iGCSE Physics and iGCSE Biology), sorry for any inconvenience, thank you for sticking with me! If you have any questions about any posts do not hesitate to comment and I will reply as soon as possibly, hope any exams you have already done went smoothly (for those doing Cambridge iGCSE english... what was the obsession with the bees?), good luck for any doing art and textiles exams over the next few days also. I will endeavour to finish the blogs as soon as possible next week so I have all the rest of study leave to answer any questions or anything you have. Good luck once more, I know you will all do amazing :)
Millie
Millie
Wednesday 27 April 2016
5.9 describe the trend in boiling point and viscosity of the main fractions
The lower the boiling point, the lower the viscosity, and vice versa (high boiling point = high viscosity)
NOTE: basically, the lower the viscosity, the thinner the substance runs, the higher the viscosity, the thicker something is (as liquid). For example, water has a super low viscosity, whilst syrup has a higher viscosity than water.
NOTE: basically, the lower the viscosity, the thinner the substance runs, the higher the viscosity, the thicker something is (as liquid). For example, water has a super low viscosity, whilst syrup has a higher viscosity than water.
5.8 recall the names and uses of the main fractions obtained from crude oil: refinery gases, gasoline, kerosene, diesel, fuel oil and bitumen
Refinery gases - used in pottery and glass manufacture, heating and bottled gas
Gasoline - used for fuel for cars (its petrol, in other words)
Naphtha - Used as a starting material of making things like plastics,dyes, drugs, explosives and paints (to name a few)
Kerosene - used to fuel jets, in heating and in paint solvents
Diesel - fuel for cars, trucks, trains and boats
Fuel oil - central heating and fuel for really big ships
Bitumen - used to surface roads
Gasoline - used for fuel for cars (its petrol, in other words)
Naphtha - Used as a starting material of making things like plastics,dyes, drugs, explosives and paints (to name a few)
Kerosene - used to fuel jets, in heating and in paint solvents
Diesel - fuel for cars, trucks, trains and boats
Fuel oil - central heating and fuel for really big ships
Bitumen - used to surface roads
5.7 describe and explain how the industrial process of fractional distillation separates crude oil into fractions
NOTE: the process of fractional distillation is mentioned in point 1.7
One use of fractional distillation is separating crude oil into fractions (compounds). Heres how it happens...
- Heat the crude oil until almost all of it has turned into gas, these gases will enter the fractioning column as they evaporate. NOTE: the left over liquid is bitumen, it has a very high boiling temperature so is instead tapped off at the bottom of the column.
- NOTE: in the column there is a temperature gradient. Basically, it is really hot at the bottom and quite bit colder in the top (not actually cold though). This is useful as the substances that make up crude oil have different boiling temperatures, when the substance meets the part of the tube where it is cooler than their boiling temperature, they will condense and drain off down a tube. (NOTE: hydrocarbons with short carbon chains have a low boiling point, so they condense near the top of the fractioning column whilst hydrocarbons with long carbon chains have high boiling points, so they condense near the bottom of the fractioning column.
- This means you result with the different fractions of crude oil (each fraction contains a mixture of hydrocarbons with similar boiling points).
One use of fractional distillation is separating crude oil into fractions (compounds). Heres how it happens...
- Heat the crude oil until almost all of it has turned into gas, these gases will enter the fractioning column as they evaporate. NOTE: the left over liquid is bitumen, it has a very high boiling temperature so is instead tapped off at the bottom of the column.
- NOTE: in the column there is a temperature gradient. Basically, it is really hot at the bottom and quite bit colder in the top (not actually cold though). This is useful as the substances that make up crude oil have different boiling temperatures, when the substance meets the part of the tube where it is cooler than their boiling temperature, they will condense and drain off down a tube. (NOTE: hydrocarbons with short carbon chains have a low boiling point, so they condense near the top of the fractioning column whilst hydrocarbons with long carbon chains have high boiling points, so they condense near the bottom of the fractioning column.
- This means you result with the different fractions of crude oil (each fraction contains a mixture of hydrocarbons with similar boiling points).
Tuesday 26 April 2016
5.6 understand that crude oil is a mixture of hydrocarbons
Hydrocarbons are molecules which contain only hydrogen and carbon atoms. Crude oil is made up of only hydrocarbons (but lots of different types of them).
5.5 explain the uses of aluminium and iron, in terms of their properties
Uses (due to properties) of iron
The properties of iron are all the usual properties of a metal. However, adding something to iron can change its properties (as expected), this means it an be made suitable for many different things. Fir example...
- Pure iron (wrought iron) is malleable ('bendable'), this means it is used to make things such as ornamental gates and railings (as it can be twisted into pretty shapes etc)
- Cast iron (a mixture of iron, carbon and silicon) is very hard, this means it is used to make things such as manhole covers (that undergo lots of pressure, from vehicles, daily) and also some cooking pans
- Steel (an alloy made of iron) is harder than pure (wrought) iron but is still malleable and can also be welded together. It can be easily hammered into sheets and, because of this, it is great for making things that need thin hard metal, such as car bodies and girders for construction.
NOTE: a downside to using iron is that it rusts easily. However, stainless steel (an alloy of iron and chromium) will not rust, because of this, it is used in making things such as cutlery and pans that are exposed to water often (when cleaning etc).
Uses (due to properties) of aluminium
Aluminium is slightly different to iron, it also has all the main properties of a metal however, it doesn't corrode easily. This is because it quickly reacts with oxygen in the air, producing aluminium oxide (which forms as a protective layer around the aluminium, stopping any further reaction taking place) - this stops corrosion.
Due to its non-corroding property, it is used to make products that often come into contact with water (such as coke cans etc).
Aluminium is also a lot less dense than iron, which consequently makes it lighter (less particles per certain area etc), this means it is useful for making things when the weight of a metal frame needs to be taken into consideration (for example, when producing a bicycle frame or aeroplane body)
The properties of iron are all the usual properties of a metal. However, adding something to iron can change its properties (as expected), this means it an be made suitable for many different things. Fir example...
- Pure iron (wrought iron) is malleable ('bendable'), this means it is used to make things such as ornamental gates and railings (as it can be twisted into pretty shapes etc)
- Cast iron (a mixture of iron, carbon and silicon) is very hard, this means it is used to make things such as manhole covers (that undergo lots of pressure, from vehicles, daily) and also some cooking pans
- Steel (an alloy made of iron) is harder than pure (wrought) iron but is still malleable and can also be welded together. It can be easily hammered into sheets and, because of this, it is great for making things that need thin hard metal, such as car bodies and girders for construction.
NOTE: a downside to using iron is that it rusts easily. However, stainless steel (an alloy of iron and chromium) will not rust, because of this, it is used in making things such as cutlery and pans that are exposed to water often (when cleaning etc).
Uses (due to properties) of aluminium
Aluminium is slightly different to iron, it also has all the main properties of a metal however, it doesn't corrode easily. This is because it quickly reacts with oxygen in the air, producing aluminium oxide (which forms as a protective layer around the aluminium, stopping any further reaction taking place) - this stops corrosion.
Due to its non-corroding property, it is used to make products that often come into contact with water (such as coke cans etc).
Aluminium is also a lot less dense than iron, which consequently makes it lighter (less particles per certain area etc), this means it is useful for making things when the weight of a metal frame needs to be taken into consideration (for example, when producing a bicycle frame or aeroplane body)
Sunday 24 April 2016
5.4 describe and explain the main reactions involved in the extraction of iron from iron ore (hematite), using one, limestone and air in a blast furnace
In order to extract iron from hematite (iron ore) you need a blast furnace, coke (for reducing the iron oxide to iron metal) and limestone (for taking away impurities). The process is as follows...

- Hot air is blasted into the furnace (hence name, blast furnace), this makes the coke burn much faster than normal, and also raises the temperature to around 1500ºC. The coke burns to produce carbon dioxide (C + O2 ---> CO2)
- The CO2 then reacts with unburnt/leftover coke, producing carbon monoxide (CO2 + C ---> 2CO)
- The carbon monoxide will then react with the iron ore, producing iron. (3CO + Fe2O3 ---> 3CO2+ 2Fe)
- The limestone removes the silicon dioxide (SiO2) that is the main impurity. This happens as the limestone is decomposed by the heat into calcium oxide and carbon dioxide (CaCO3 ---> CaO + CO2). The calcium oxide then reacts with the silicon dioxide forming calcium silicate, aka slag (CaO + SiO2---> CaSiO3).
- The iron and slag are both molten so sink to the bottom of the furnace. However, slag is less dense than iron so the slag sits into of the iron, they are both tapped off.
NOTE: although the slag is useless in this, the process is still sustainable as the slag is not wasted. It can be used in fertilisers and road building (bit random, i know)
NOTE NOTE: It is very important to understand that this is a reduction reaction (the iron is reduced as it loses oxygen)
image credit: BBC
- Hot air is blasted into the furnace (hence name, blast furnace), this makes the coke burn much faster than normal, and also raises the temperature to around 1500ºC. The coke burns to produce carbon dioxide (C + O2 ---> CO2)
- The CO2 then reacts with unburnt/leftover coke, producing carbon monoxide (CO2 + C ---> 2CO)
- The carbon monoxide will then react with the iron ore, producing iron. (3CO + Fe2O3 ---> 3CO2+ 2Fe)
- The limestone removes the silicon dioxide (SiO2) that is the main impurity. This happens as the limestone is decomposed by the heat into calcium oxide and carbon dioxide (CaCO3 ---> CaO + CO2). The calcium oxide then reacts with the silicon dioxide forming calcium silicate, aka slag (CaO + SiO2---> CaSiO3).
- The iron and slag are both molten so sink to the bottom of the furnace. However, slag is less dense than iron so the slag sits into of the iron, they are both tapped off.
NOTE: although the slag is useless in this, the process is still sustainable as the slag is not wasted. It can be used in fertilisers and road building (bit random, i know)
NOTE NOTE: It is very important to understand that this is a reduction reaction (the iron is reduced as it loses oxygen)
image credit: BBC
Saturday 23 April 2016
5.3 write ionic half-equations for the reactions at the electrodes in aluminium extraction.
If you are looking at this post I assume you know what the electrolysis of aluminium oxide is and the implications, if you do not know, this may help... 5.2
The equations are as follows...
At the anode (positive electrode): 2O2− ---> O2 + 4e−
The equations are as follows...
At the anode (positive electrode): 2O2− ---> O2 + 4e−
At the cathode (negative electrode): Al3+ + 3e− ---> Al
5.2 describe and explain the extraction of aluminium from purified aluminium oxide by electrolysis including: i the use of molten cryolite as a solvent and to decrease the required operating temperature ii the need to replace the positive electrodes iii the cost of the electricity as a major factor
As aluminium is more reactive than carbon, you use electrolysis to extract aluminium from its ore. The main ore of aluminium is bauxite (which is mined, incase you were wondering). NOTE: after you purify bauxite, a white powder is left (this is aluminium oxide, Al2O3).
First lets start with the electrolysis process, if you are familiar with this just skip to 'problems'...
1- Aluminium oxide is melted (to form molten aluminium oxide), this contains free ions, meaning it will conduct electricity.
2- The positive Al3+ ions are attracted to the negative electrode (the cathode, this is lining the electrolysis cell). Here, the positive Al3+ ions gain electrons (3 to be precise, to balance their charge), and they turn into aluminium atoms, which sinks to the bottom as molten aluminium.
3- The negative O2− ions are attractive to the positive electrodes (anodes). Are, they lose electrons. They will then react together to form O2 (oxygen gas), which will occasionally react with the anodes forming carbon dioxide (CO2).
NOTE: this is a redox reaction as reduction and oxidation both take place. The equations are as follows...
at the cathode: Al3+ + e− ---> Al
at the anode: 2O2− ---> O2 + 4e−
The complete decomposition reaction: aluminium oxide ---> aluminium + oxygen
Problems
However, the melting point of Al2O3 is very high (around 2000ºC, if you were wondering). Aluminium oxide is dissolved in molten cryolite (a less common ore of aluminium), this lowers the temperature needed to melt, so less fuel is used in heating the aluminium oxide = saves energy. (NOTE: using cryolite only lowers the melting temperature to around 900ºC so, although it lowers the temperature and saves lots of energy (and also makes the extraction cheaper and easier), a very high temp is still needed.
The positive electrodes (anodes) are made of graphite (a form of carbon). During the electrolysis process, the O2− ions react with the carbon anodes, forming carbon dioxide (CO2). This means that the anodes will eventually have to be replaced, as they will start to erode where they react with oxygen.
Lots of energy is needed to heat the aluminium oxide to 900ºC and therefore electrolysis of aluminium is very expensive. Furthermore, electrolysis itself uses lots of electricity.
5.1 explain how the methods of extraction of the metals in this section are related to their positions in the reactivity series
Methods of extraction are closely linked to the position of a metal in the reactivity series. This means that, by looking at the reactivity series, you can easily see the best way to extract that metal. They are grouped as follows...
All (and only) metals below carbon in the reactivity series (zinc, iron and tin)can be extracted by a reduction reaction with carbon (method: heat the ore with carbon monoxide). This happens because the carbon is more reactive, so for example (when extracting iron from iron oxide) the carbon displaces the iron, to form carbon dioxide and iron (NOTE: this is why we react carbon monoxide, so carbon dioxide is formed, if we were to react just carbon, carbon monoxide would we be formed which is poisonous).
If an element is more reactive than carbon (higher than carbon in the reactivity series), carbon will not displace it. This means that all metals more reactive than carbon have to be displaced with electrolysis (this separates the metal from the other elements in the compound using electricity).
All (and only) metals below carbon in the reactivity series (zinc, iron and tin)can be extracted by a reduction reaction with carbon (method: heat the ore with carbon monoxide). This happens because the carbon is more reactive, so for example (when extracting iron from iron oxide) the carbon displaces the iron, to form carbon dioxide and iron (NOTE: this is why we react carbon monoxide, so carbon dioxide is formed, if we were to react just carbon, carbon monoxide would we be formed which is poisonous).
If an element is more reactive than carbon (higher than carbon in the reactivity series), carbon will not displace it. This means that all metals more reactive than carbon have to be displaced with electrolysis (this separates the metal from the other elements in the compound using electricity).
image credit: slideshare
Wednesday 20 April 2016
4.25 predict the effects of changing the pressure and temperature on the equilibrium position in reversible reactions
NOTE: It is important to remember in reversible reactions one reaction is endothermic and the other is exothermic.
The temperature and pressure of the reactants have a very strong effect on the position of the equilibrium. For example...
Temperature
If you increase the temperature - the endothermic reaction will increase to use up the heat
If you decrease the temperature - the exothermic reaction will increase to raise the heat
Pressure
If you increase the pressure - the reaction will produce the side of the equation that has the least moles (to work this out, just look at the ratio of moles on either side of the equation)
If you decrease the pressure - the reaction will produce the side of the equation that has the most amount of moles
The temperature and pressure of the reactants have a very strong effect on the position of the equilibrium. For example...
Temperature
If you increase the temperature - the endothermic reaction will increase to use up the heat
If you decrease the temperature - the exothermic reaction will increase to raise the heat
Pressure
If you increase the pressure - the reaction will produce the side of the equation that has the least moles (to work this out, just look at the ratio of moles on either side of the equation)
If you decrease the pressure - the reaction will produce the side of the equation that has the most amount of moles
4.24 understand the concept of dynamic equilibrium
Okay so this may need a few reads as it is a little complicated but this was the easiest way i could explain it... good luck :)
If a reversible reactions occurs (in a closed system), a dynamic equilibrium will be reached. All this means is that the relative % of reactants and products will reach a balance and stay there (NOTE: it is not always 50-50), the reactions will then continue to occur (basically the reaction takes place both ways, at the same time BUT there is no overall effect (one being made more than the other) as the relative % of reactants to products is balanced (remember :D) so the reactions kind of cancel each other out (as both reactions occur at the same rate)
NOTE: A closed system just means that none of the products/reactants can escape (this enables dynamic equilibrium to occur)
If a reversible reactions occurs (in a closed system), a dynamic equilibrium will be reached. All this means is that the relative % of reactants and products will reach a balance and stay there (NOTE: it is not always 50-50), the reactions will then continue to occur (basically the reaction takes place both ways, at the same time BUT there is no overall effect (one being made more than the other) as the relative % of reactants to products is balanced (remember :D) so the reactions kind of cancel each other out (as both reactions occur at the same rate)
NOTE: A closed system just means that none of the products/reactants can escape (this enables dynamic equilibrium to occur)
Saturday 16 April 2016
4.23 describe reversible reactants such as the dehydration of hydrated copper(II) sulfate and the effect of heat on ammonium chloride
Dehydration of hydrated copper(II) sulfate
Copper(II) sulfate is a white solid. When you added water to copper(II) sulfate it forms blue crystals, forming hydrated copper(II) sulfate. If heat this hydrated copper(II) sulfate it turns white as the water evaporates, forming (dehydrated) copper(II) sulfate. If you add water, it will turn blue again, if you heat it again it will turn white... etc
Ammonium chloride
Ammonium chloride is a white solid, when it is heated it breaks down into ammonia gas and hydrogen chloride gas. However, if you let these products (ammonia gas and hydrogen chloride) cool down, they will react with each other, forming ammonium chloride.
Copper(II) sulfate is a white solid. When you added water to copper(II) sulfate it forms blue crystals, forming hydrated copper(II) sulfate. If heat this hydrated copper(II) sulfate it turns white as the water evaporates, forming (dehydrated) copper(II) sulfate. If you add water, it will turn blue again, if you heat it again it will turn white... etc
Ammonium chloride
Ammonium chloride is a white solid, when it is heated it breaks down into ammonia gas and hydrogen chloride gas. However, if you let these products (ammonia gas and hydrogen chloride) cool down, they will react with each other, forming ammonium chloride.
4.22 understand that some reactions are reversible and are indicated by the symbol ⇌ in equations
-
- ⇌ is the symbol for reversible reaction
- NOTE: a reversible reaction is just a reaction where the products of the reaction can themselves react to produce the original reactants
4.19 understand the term activation energy and represent it on a reaction profile
The activation energy is just the minimum amount of energy the reactants needs for the reaction to start
4.21 explain that a catalyst speeds up a reaction by providing an alternative pathway with lower activation energy
Catalysts work by lowering the activation energy (the minimum energy required by reacting particles for the reaction to occur). This happens because a catalyst provides an alternative reaction pathway with a lower activation energy.
NOTE: with a catalyst, although the activation energy lowers, the enthalpy change (ΔH) is not affected.
NOTE: with a catalyst, although the activation energy lowers, the enthalpy change (ΔH) is not affected.
Tuesday 5 April 2016
4.18 describe the effects of changes in surface area of a solid, concentration of solutions, pressure of gases, temperature and the use of a catalyst on the rate of reaction
NOTE: this point is just about the changes that occur, if you want/need to understand why these changes occur, go to this point... 4.20
Changes in surface area
The bigger the surface area (to volume ratio) the faster the reaction. This is because there are more particles on the surface for the reactants to react with. In a solid, to increase the surface area without decreasing the mass just break up the solid into smaller pieces.
Concentration of solutions
If a solution is very concentrated there are lots of particles for its volume (the particles are close together). Alternatively, if the concentration is very weak there are a very little amount of particles for its volume (the particles are very spaced out).
Pressure of gases
This is very similar to concentration of solutions. If the gas is at high pressure there will be more particles squished into a certain space, more particles means a faster rate of reaction. Alternatively, low pressure results in little amount of particles meaning a slower rate of reaction.
Temperature
The hotter the reactants the faster the reaction. Alternatively, the colder the temperature of the reactants the slower the reaction rate. This is because the particles have very little energy.
Catalyst
A catalyst works by giving the reactants a surface to 'stick' to. This will increase the rate of reaction.
Changes in surface area
The bigger the surface area (to volume ratio) the faster the reaction. This is because there are more particles on the surface for the reactants to react with. In a solid, to increase the surface area without decreasing the mass just break up the solid into smaller pieces.
Concentration of solutions
If a solution is very concentrated there are lots of particles for its volume (the particles are close together). Alternatively, if the concentration is very weak there are a very little amount of particles for its volume (the particles are very spaced out).
Pressure of gases
This is very similar to concentration of solutions. If the gas is at high pressure there will be more particles squished into a certain space, more particles means a faster rate of reaction. Alternatively, low pressure results in little amount of particles meaning a slower rate of reaction.
Temperature
The hotter the reactants the faster the reaction. Alternatively, the colder the temperature of the reactants the slower the reaction rate. This is because the particles have very little energy.
Catalyst
A catalyst works by giving the reactants a surface to 'stick' to. This will increase the rate of reaction.
4.20 explain the effects of changes in surface area of a solid, concentration of solutions, pressure of gases and temperature on the rate of reaction in terms of partial collision theory
Changes in surface area
The bigger the surface area (to volume ratio) the faster the reaction. This is because there are more particles on the surface for the reactants to react with, meaning more collisions with the particles meaning a faster reaction. In a solid, to increase the surface area without decreasing the mass just break up the solid into smaller pieces.
Concentration of solutions
If a solution is very concentrated there are lots of particles for its volume (the particles are close together). More particles means more collisions and therefore a faster reaction rate. Alternatively, if the concentration is very weak there are a very little amount of particles for its volume (the particles are very spaced out). This means collisions will be less frequent (are there are less particles to collide with) so the reaction is slower.
Pressure of gases
This is very similar to concentration of solutions. If the gas is at high pressure there will be more particles squished into a certain space, more particles mean more collisions which mean a faster rate of reaction. Alternatively, low pressure results in little amount of particles in a certain space meaning less collisions (as there are not as many particles to collide with) meaning a slower rate of reaction.
Temperature
The hotter the reactants the faster the reaction. This is because the particles have more energy due to the heat. Because they have more energy this means they will more faster and therefore collide more. Alternatively, the colder the temperature of the reactants the slower the reaction rate. This is because the particles have very little energy.
NOTE: faster collisions are only increased by temperature, all other ways to increase reaction rate increase the amount of particles to collide with.
The bigger the surface area (to volume ratio) the faster the reaction. This is because there are more particles on the surface for the reactants to react with, meaning more collisions with the particles meaning a faster reaction. In a solid, to increase the surface area without decreasing the mass just break up the solid into smaller pieces.
Concentration of solutions
If a solution is very concentrated there are lots of particles for its volume (the particles are close together). More particles means more collisions and therefore a faster reaction rate. Alternatively, if the concentration is very weak there are a very little amount of particles for its volume (the particles are very spaced out). This means collisions will be less frequent (are there are less particles to collide with) so the reaction is slower.
Pressure of gases
This is very similar to concentration of solutions. If the gas is at high pressure there will be more particles squished into a certain space, more particles mean more collisions which mean a faster rate of reaction. Alternatively, low pressure results in little amount of particles in a certain space meaning less collisions (as there are not as many particles to collide with) meaning a slower rate of reaction.
Temperature
The hotter the reactants the faster the reaction. This is because the particles have more energy due to the heat. Because they have more energy this means they will more faster and therefore collide more. Alternatively, the colder the temperature of the reactants the slower the reaction rate. This is because the particles have very little energy.
NOTE: faster collisions are only increased by temperature, all other ways to increase reaction rate increase the amount of particles to collide with.
4.17 describe experiments to investigate the effects of changes in surface area of a solid, concentration of solutions, temperature and the use of a catalyst on the rate of reaction
Changes in surface area
A change in surface area will affect the rate of reaction as there will be more/less surface area, meaning more/less collisions with more/less particles.
Method
- Measure out 50g of large pieces of marble chips and put them in a conical flask
- Add 50ml of dilute HCl
- Straight after you add the acid, put a bung in the top and attach it to a delivery tube attached to a gas syringe and start a stopwatch
- For 5 minutes, and at 30 second intervals, record the volume of gas collected in the syringe.
- Plot your results on a graph with time (the independent variable) as x and volume of gas collected (the dependant variable) as y.
- Repeat this experiment with smaller marble chips (still the same amount of mass... 50g).
- Now repeat again with powdered marble chips
- Plot these results on the same graph for easy comparison
Conclusion
Should all go well, you should conclude that the experiment using powdered marble chips produced the most amount of CO2 in the same amount of time This is because an increased surface area causes more collisions, therefore the rate of reaction is faster, therefore more gas is produced.
NOTE: The gas given off is CO2, remember this is a small scale version of how to make CO2 in a laboratory, if you are unsure of how this is done, here it is... 2.20 :)
Changes in concentration
A change in concentration will affect the rate of reaction as there will be more particles to collide, so the reaction will be completed quicker
Method
- Measure 50g of magnesium metal and put it in a conical flask
- Put the flask on scales
- Add 50ml of least concentrated HCl (do not remove flask from scales)
- Record the mass of the flask with magnesium and HCl then immediately start a stopwatch
- Record the mass of the experiment for 5 minutes, at 30 second intervals
- Plot your results on a graph with time (the independent variable) as x and mass loss (the dependant variable) as y. NOTE: the mass will decrease as this reaction produces hydrogen gas, which 'floats' away.
- Repeat this experiment with different concentrations of HCl (ensure to keep the same mass of magnesium...50g and volume of acid...50ml) and plot your results on the same graph
Conclusion
You should find that the higher the concentration the steeper the graph (the quicker the reaction).
A change in surface area will affect the rate of reaction as there will be more/less surface area, meaning more/less collisions with more/less particles.
Method
- Measure out 50g of large pieces of marble chips and put them in a conical flask
- Add 50ml of dilute HCl
- Straight after you add the acid, put a bung in the top and attach it to a delivery tube attached to a gas syringe and start a stopwatch
- For 5 minutes, and at 30 second intervals, record the volume of gas collected in the syringe.
- Plot your results on a graph with time (the independent variable) as x and volume of gas collected (the dependant variable) as y.
- Repeat this experiment with smaller marble chips (still the same amount of mass... 50g).
- Now repeat again with powdered marble chips
- Plot these results on the same graph for easy comparison
Conclusion
Should all go well, you should conclude that the experiment using powdered marble chips produced the most amount of CO2 in the same amount of time This is because an increased surface area causes more collisions, therefore the rate of reaction is faster, therefore more gas is produced.
NOTE: The gas given off is CO2, remember this is a small scale version of how to make CO2 in a laboratory, if you are unsure of how this is done, here it is... 2.20 :)
Changes in concentration
A change in concentration will affect the rate of reaction as there will be more particles to collide, so the reaction will be completed quicker
Method
- Measure 50g of magnesium metal and put it in a conical flask
- Put the flask on scales
- Add 50ml of least concentrated HCl (do not remove flask from scales)
- Record the mass of the flask with magnesium and HCl then immediately start a stopwatch
- Record the mass of the experiment for 5 minutes, at 30 second intervals
- Plot your results on a graph with time (the independent variable) as x and mass loss (the dependant variable) as y. NOTE: the mass will decrease as this reaction produces hydrogen gas, which 'floats' away.
- Repeat this experiment with different concentrations of HCl (ensure to keep the same mass of magnesium...50g and volume of acid...50ml) and plot your results on the same graph
Conclusion
You should find that the higher the concentration the steeper the graph (the quicker the reaction).
Changes in temperature
The higher the temperature the faster the reaction, this is because the particles have more energy from the heat.
Method
- Draw an X (or any clear shape) on a piece of paper and put a conical flask on top
- Add 50ml of sodium thiosulphate and 50ml of HCl and immediately start a stopwatch
- Time how long it takes for the X to 'disappear' (NOTE: it will become not visible as this reaction produced a yellow precipitate of sulfur which cloudy the flask so you can't see the X, just time how long it takes until you can't see the X).
- repeat the experiment but with the two solutions at a higher temperature (using a water bath to heat the two solutions before adding them together).
- Repeat 5 times, increasing the heat of the solutions each time.
- Plot your result in a table for easy comparison.
Conclusion
This reaction should show that the higher the temperature, the quicker the X disappears, therefore the quicker the reaction.
Using a catalyst (decomposition of hydrogen peroxide)
The use of a catalyst will increase the rate of reaction.
Method
- Add 50ml of hydrogen peroxide to a conical flask
- Put a bung on top and attach it to a delivery tube attached to a gas syringe
- Start a stopwatch
- Time how much gas is collected (in the gas syringe) in 10 minutes at 30 second intervals (probably VERY little as the reaction naturally is very slow)
- Plot your results on a graph with time (the independent variable) as x and volume of gas collected (the dependant variable) as y.
- Repeat experiment, but add in a bit of manganese (IV) oxide (this is a catalyst)
- Repeat this experiment with smaller marble chips (still the same amount of mass... 50g).
- Now repeat again with powdered marble chips
- Plot these results on the same graph for easy comparison
- Put a bung on top and attach it to a delivery tube attached to a gas syringe
- Start a stopwatch
- Time how much gas is collected (in the gas syringe) in 10 minutes at 30 second intervals (probably VERY little as the reaction naturally is very slow)
- Plot your results on a graph with time (the independent variable) as x and volume of gas collected (the dependant variable) as y.
- Repeat experiment, but add in a bit of manganese (IV) oxide (this is a catalyst)
- Repeat this experiment with smaller marble chips (still the same amount of mass... 50g).
- Now repeat again with powdered marble chips
- Plot these results on the same graph for easy comparison
Incase you were wondering, the equation for this reaction is...
2H2O2(aq) ---> 2H2O(l) + O2 (g)
NOTE: The gas given off is oxygen, remember this is a small scale version of how to make oxygen in a laboratory, if you are unsure of how this is done, here it is... 2.18 :)
Monday 4 April 2016
4.16 use average bond energies to calculate the enthalpy change during a simple chemical reaction
Each type of bond (for example O-O or C-H) has a particular bond energy... we will be given these in the exam so don't worry about learning them. These energies can be used to calculate enthalpy change, for example...
Using bond energies, calculate the enthalpy change for the following reaction
H2 + Cl2 ---> 2HCl
Bond energies...
H-H: +436 kJ/mol
Cl-Cl: +242 kJ/mol
H-Cl: +431kJ/mol
1. Work out what bonds are broken & the energy made...
1 mole of H-H is broken and 1 mole of Cl-Cl is broken. Therefore, +436 + +242 = +678 kJ/mol is required to break the bonds in this reaction
2. Work out what bonds are being made & the energy released...
Forming 2 moles of H-Cl bonds. This released 2 x +431 = 862kJ/mol
3. Use the formula 'ΔH = total energy absorbed to break bonds - total energy released in making bonds' to find out the enthalpy change
ΔH = 678 - 862 = -184 kJ/mol
4. ΔH is negative which means the reaction must be exothermic
Using bond energies, calculate the enthalpy change for the following reaction
H2 + Cl2 ---> 2HCl
Bond energies...
H-H: +436 kJ/mol
Cl-Cl: +242 kJ/mol
H-Cl: +431kJ/mol
1. Work out what bonds are broken & the energy made...
1 mole of H-H is broken and 1 mole of Cl-Cl is broken. Therefore, +436 + +242 = +678 kJ/mol is required to break the bonds in this reaction
2. Work out what bonds are being made & the energy released...
Forming 2 moles of H-Cl bonds. This released 2 x +431 = 862kJ/mol
3. Use the formula 'ΔH = total energy absorbed to break bonds - total energy released in making bonds' to find out the enthalpy change
ΔH = 678 - 862 = -184 kJ/mol
4. ΔH is negative which means the reaction must be exothermic
4.15 understand that the breaking of bonds is endothermic and hat the making of bonds is exothermic
Not much to say here... when you make a bond it is an exothermic reaction, when you break a bond it is an endothermic reaction.
Energy must be supplied to break existing bonds, so bond breaking is endothermic. However, energy is released when new bonds are formed, so bond formation is exothermic.
Energy must be supplied to break existing bonds, so bond breaking is endothermic. However, energy is released when new bonds are formed, so bond formation is exothermic.
4.14 represent exothermic and endothermic reactions on a simple energy level diagram
In exothermic reactions
 This energy level diagram shows an exothermic reaction, we can tell this because the products are at a lower energy level than the reactants. The difference in height (ΔH) represents the energy given out in the reaction. ΔH is negative here because the reaction is giving out energy (as it is an exothermic reaction). The activation energy represents the energy needed to break the old bonds.
This energy level diagram shows an exothermic reaction, we can tell this because the products are at a lower energy level than the reactants. The difference in height (ΔH) represents the energy given out in the reaction. ΔH is negative here because the reaction is giving out energy (as it is an exothermic reaction). The activation energy represents the energy needed to break the old bonds.
In endothermic reactions

 This energy level diagram shows an exothermic reaction, we can tell this because the products are at a lower energy level than the reactants. The difference in height (ΔH) represents the energy given out in the reaction. ΔH is negative here because the reaction is giving out energy (as it is an exothermic reaction). The activation energy represents the energy needed to break the old bonds.
This energy level diagram shows an exothermic reaction, we can tell this because the products are at a lower energy level than the reactants. The difference in height (ΔH) represents the energy given out in the reaction. ΔH is negative here because the reaction is giving out energy (as it is an exothermic reaction). The activation energy represents the energy needed to break the old bonds.In endothermic reactions

This energy level diagram shown an endothermic reaction, we can tell this because the products are at a higher energy level than the reactants. The difference in height (ΔH) represents the energy taken. ΔH is positive because the reaction is taking in energy (because is an endothermic reaction).
Basis of notes source: CGP
Basis of notes source: CGP
4.13 understand the use of ΔH to represent enthalpy change for exothermic and endothermic reactions
The enthalpy change is the overall change in energy in a reaction, it is symbolised by ΔH and its unit is kJ/mol, as it is the amount of energy in kilojoules per mole of reactant. It can be positive or negative, if the reaction is exothermic, the enthalpy change is negative because the reaction gives out energy, if the reaction is endothermic, the enthalpy change value is positive because the reaction takes in energy.
4.12 Calculate molar enthalpy change from heat energy change
Okay so you have calculated the amount of energy produced , this can be used to work out the molar enthalpy change (this is basically the enthalpy change given out by one mole of the reactant).
NOTE:
To calculate the molar enthalpy change, you need to know the equations moles = mass / Mr (if unsure of this equation, click here) and molar enthalpy change = energy produced / moles. For example...
0.9g of methylated spirit produces 6510J of heat energy, work out the heat produced per mole. (The Mr of methylated spirit is 44.6)
- First, work out the amount of energy transferred...
We know that 6510J of heat energy was produced, this means 6510J of energy was transferred... this needs to be converted into kJ (as the unit or enthalpy change is kJ/mol)... 6.510kJ
- Next, find out how many moles of fuel produced this heat...
moles = mass / Mr
= 0.9 / 44.6
= 0.02 moles
- Now, divide the amount of heat energy produced by the amount of moles...
6.510 / 0.02 = 325.5 kJ/mol
The end (:
NOTE:
To calculate the molar enthalpy change, you need to know the equations moles = mass / Mr (if unsure of this equation, click here) and molar enthalpy change = energy produced / moles. For example...
0.9g of methylated spirit produces 6510J of heat energy, work out the heat produced per mole. (The Mr of methylated spirit is 44.6)
- First, work out the amount of energy transferred...
We know that 6510J of heat energy was produced, this means 6510J of energy was transferred... this needs to be converted into kJ (as the unit or enthalpy change is kJ/mol)... 6.510kJ
- Next, find out how many moles of fuel produced this heat...
moles = mass / Mr
= 0.9 / 44.6
= 0.02 moles
- Now, divide the amount of heat energy produced by the amount of moles...
6.510 / 0.02 = 325.5 kJ/mol
The end (:
4.11 describe simple calorimetry experiments for reactions such as combustion, displacement, dissolving and neutralisation in which heat energy changes can be calculated from measured temperature changes
NOTE: THIS IS SO CONFUSING I DON'T UNDERSTAND WHATS GOING ON the only example/explanation for this I could find was in the CGP revision guide but I still don't understand it so will defiantly be coming back to this post to edit it after i have finished the spec, if anyone knows how to do it or has any info or anything please comment it and I will add it to the post!
Combustion
This is basically the same method as 2.32 of the biology spec but with fuel not food... to measure the amount of energy produced when a fuel is burnt, just burn the fuel and use the flame to heat up some water...
- Put 50g of water into a copper can (because copper is a very good conductor of heat)
- Record the temperature of the water
- Weigh the spirit burner and lid (the spirit burner contains the fuel)
- Place the spirit burner underneath the copper can and light its wick.
- Stir constantly until the water reaches about 50ºC
- Put the flame out using the burner lid
- Record the final temperature of the water
- Weigh the spirit burner and lid again
- calculate the enthalpy change
Your setup should look something like this...

Dissolving, displacement and neutralisation reactions
Combustion
This is basically the same method as 2.32 of the biology spec but with fuel not food... to measure the amount of energy produced when a fuel is burnt, just burn the fuel and use the flame to heat up some water...
- Put 50g of water into a copper can (because copper is a very good conductor of heat)
- Record the temperature of the water
- Weigh the spirit burner and lid (the spirit burner contains the fuel)
- Place the spirit burner underneath the copper can and light its wick.
- Stir constantly until the water reaches about 50ºC
- Put the flame out using the burner lid
- Record the final temperature of the water
- Weigh the spirit burner and lid again
- calculate the enthalpy change
Your setup should look something like this...

NOTE: the draught shield is just there to ensure no/little heat escapes
Dissolving, displacement and neutralisation reactions
4.9 describe experiments to carry out acid-alkali titrations
By doing a titration you are able to find out exactly how much acid is needed to neutralise a certain amount of alkali, exactly how much alkali is needed to neutralise a certain amount of acid.
Method
- Add 25cm3 of alkali to a conical flask (with a pipette and pipette filler)
- Add a few drops of phenolphthalein indicator
- Fill a burette with your acid (NOTE: ensure you have the burette below eye level incase the acid sprays out etc)
- Add the acid to the alkali a bit at a time (using a burette). NOTE: regularly swirl the conical flask to ensure the acid is evenly distributed throughout the alkaline
- Stop adding acid as soon as the solution in the conical flask changes colour (with phenolphthalein, it will go colourless) - this means that the alkali has been neutralised.
- Using the burette, record the amount of acid used to neutralise the alkali.
- Repeat a few times to avoid anomalies etc.
If you are asked to find out the amount in moles of acid needed to neutralise an alkali (or vice versa), just do the same as a mole calculation. For example...
It takes 30cm3 of sulphuric acid of an unknown concentration to neutralise 25cm3 of sodium hydroxide with a concentration of 0.1 moles per dm3. Find the concentration of the sulphuric acid.
- First, work out how many moles of the 'known' substance (sodium hydroxide) you have...
moles = concentration x volume
= 0.1 x (25/1000)
= 0.0025 moles
- Now, write down the equation to work out the ratio of acid : alkali...
2NaOH + H2SO4 ---> Na2SO4 + 2H2O
This means there are two moles of sodium hydroxide to every 1 mole of sulphuric acid.
- This means we need to half the amount of moles of sodium hydroxide to get the amount of moles of sulphuric acid...
0.0025/2 = 0.00125
- Now we know the amount of moles and volume of the acid, so we can work out the concentration...
concentration = moles / volume
= 0.00125 / (30/1000)
= 0.0417 moles per dm3
Method
- Add 25cm3 of alkali to a conical flask (with a pipette and pipette filler)
- Add a few drops of phenolphthalein indicator
- Fill a burette with your acid (NOTE: ensure you have the burette below eye level incase the acid sprays out etc)
- Add the acid to the alkali a bit at a time (using a burette). NOTE: regularly swirl the conical flask to ensure the acid is evenly distributed throughout the alkaline
- Stop adding acid as soon as the solution in the conical flask changes colour (with phenolphthalein, it will go colourless) - this means that the alkali has been neutralised.
- Using the burette, record the amount of acid used to neutralise the alkali.
- Repeat a few times to avoid anomalies etc.
If you are asked to find out the amount in moles of acid needed to neutralise an alkali (or vice versa), just do the same as a mole calculation. For example...
It takes 30cm3 of sulphuric acid of an unknown concentration to neutralise 25cm3 of sodium hydroxide with a concentration of 0.1 moles per dm3. Find the concentration of the sulphuric acid.
- First, work out how many moles of the 'known' substance (sodium hydroxide) you have...
moles = concentration x volume
= 0.1 x (25/1000)
= 0.0025 moles
- Now, write down the equation to work out the ratio of acid : alkali...
2NaOH + H2SO4 ---> Na2SO4 + 2H2O
This means there are two moles of sodium hydroxide to every 1 mole of sulphuric acid.
- This means we need to half the amount of moles of sodium hydroxide to get the amount of moles of sulphuric acid...
0.0025/2 = 0.00125
- Now we know the amount of moles and volume of the acid, so we can work out the concentration...
concentration = moles / volume
= 0.00125 / (30/1000)
= 0.0417 moles per dm3
4.10 understand that chemical reactions in which heat energy is given out are described as exothermic and those in which heat energy is taken in are endothermic
NOTE: before you get your head round endothermic and exothermic, it might be a good idea to understand that energy is given out when bonds are made, and taken in when bonds are destroyed...
If a reaction gives out heat, it is said to be exothermic and the energy released in bond formation is greater than the energy used in breaking bonds.
Definition for exams: An exothermic reaction is one which gives out energy to the surroundings, usually in the form of heat and usually shown by a rise in temperature.
If a reaction is endothermic, it takes in heat from its surroundings during the reaction process. This is because the energy required to break old bonds is greater than the energy released when new bonds are formed.
Definition for exams: An endothermic reaction is one which takes in energy from the surroundings, usually in the form of heat and usually shown by a fall in temperature.
If a reaction gives out heat, it is said to be exothermic and the energy released in bond formation is greater than the energy used in breaking bonds.
Definition for exams: An exothermic reaction is one which gives out energy to the surroundings, usually in the form of heat and usually shown by a rise in temperature.
If a reaction is endothermic, it takes in heat from its surroundings during the reaction process. This is because the energy required to break old bonds is greater than the energy released when new bonds are formed.
Definition for exams: An endothermic reaction is one which takes in energy from the surroundings, usually in the form of heat and usually shown by a fall in temperature.
4.8 describe experiments to prepare insoluble salts using precipitation reactions
You can use a precipitation reaction. To do this just pick two solutions that contains the ions you need and add them together. For example, to make barium sulfate (insoluble) you need to add together one solution containing barium ions and another containing sulphate ions. To do this, you cam mix barium chloride with sulphuric acid...
barium chloride + sulphuric acid ---> barium sulphate + hydrochloric acid
BaCl2(aq) + H2SO4(aq) ---> BaSO4(s) + 2HCl(aq)
barium chloride + sulphuric acid ---> barium sulphate + hydrochloric acid
BaCl2(aq) + H2SO4(aq) ---> BaSO4(s) + 2HCl(aq)
4.7 describe experiments to prepare soluble salts from acids
You need to pick an insoluble base, and the acid of the salt you want to make (sorry that was a bit confusing, I couldn't really find another way to word it). Basically, if you was to make copper nitrate, mix nitric acid and copper carbonate (remember all carbonates are insoluble except sodium, potassium and ammonium carbonate)...
CuCO3(s) + 2HNO3(aq) ---> Cu(NO3)2(aq) + CO2(g) + H2O(l)
All you need to do is add the metal oxide, carbonate or hydroxide (aka the insoluble base) to the acid. The base will react with the acid and dissolve.
NOTE: This is a neutralisation reaction, you will know when the reaction is over as this is when all the acid has been neutralised (this is when the excess solid will sink to the bottom of the flask and not react anymore)
Next, filter the solution to get rid of the undissolved base and evaporate the water to leave pure salt crystals.
CuCO3(s) + 2HNO3(aq) ---> Cu(NO3)2(aq) + CO2(g) + H2O(l)
All you need to do is add the metal oxide, carbonate or hydroxide (aka the insoluble base) to the acid. The base will react with the acid and dissolve.
NOTE: This is a neutralisation reaction, you will know when the reaction is over as this is when all the acid has been neutralised (this is when the excess solid will sink to the bottom of the flask and not react anymore)
Next, filter the solution to get rid of the undissolved base and evaporate the water to leave pure salt crystals.
Subscribe to:
Posts (Atom)




