Half equations just show what happened at each electrode. Like any equation, they need to be balanced. here are some examples...
2Br- > Br2 + 2e-
Above is an equation of a reaction at an anode. We can tell this because the Br2 loses an electron. This can be inferred as on the left it is 2Br- and on the right it is Br2 (meaning it has lost an electrode as it no longer is a negative ion, it is an atom). As it is 2Br-, 2 electrons must be lost.
2H+ + 2e- > H2
Above is an equation of a reaction at a cathode as 2H+ gains 2 electrons to become H2. The charges cancel out and it is neutral on both sides. As there are 2 'H's, there must be 2 electrons gained.
A blog covering and explaining the Edexcel IGCSE Chemistry specification for the 2016 summer exams. If you are doing just double science, you do not need to learn the stuff for paper two, if you are doing triple you will need to learn all (GOOD LUCK!) I have separated the papers to make files easier to find. Hope it helps :)
Showing posts with label Section 1. Show all posts
Showing posts with label Section 1. Show all posts
Wednesday, 18 May 2016
1.46 understand that a metal can be described as a giant structuree of positive ions surrounded by a sea of delocalised electrons
In a metal, the atoms are ionically bonded in a giant 3-D structure. The outer shell electrons become detached (making positive ions, cations). The cations are 'floating' in a sea of delocalised electrons. The metal structure stays together because the cations (+) are attracted to the electrons (-). This is known as a metallic bond.
1.31 deduce the charge of an ion from the electronic configuration of the atom from which the ion is formed
An atom with less then four electrons on its outer shell will want to lose electrons because that is the quickest way for it to have a full outer shell. So we know the atom will lose electrons (this makes positive ions).
Atoms with more than four electrons will gain electrons to fill their outer shell (as it is easier than losing electrons). This will result in negative ions.
Here are some examples...
Na has the electronic configuration of 2.8.1 (it has 1 electron on it's outer shell, so it is easiest to lose 1 electron to make a full outer shell). This results in the positive ion (a cation), Na+, which has the electronic configuration of 2.8
Cl has the electronic configuration of 2.8.7 (it has 7 electrons on it's outer shell, so it is easiest to gain 1 electron to make a full outer shell). This results in the negative ion (an anion), Cl-, which has the electronic configuration of 2.8.8
Cl has the electronic configuration of 2.8.7 (it has 7 electrons on it's outer shell, so it is easiest to gain 1 electron to make a full outer shell). This results in the negative ion (an anion), Cl-, which has the electronic configuration of 2.8.8
Tuesday, 22 March 2016
1.54 describe experiments to investigate electrolysis, using inert electrodes, of aqueous solutions such as sodium chloride, copper(II) sulphate and dilute sulphuric acid and predict the products
In aqueous solutions, as well as ions from the ionic compound, there will be hydrogen ions (H+) and hydroxide ions (OH-) from the water.
Products
At the cathode, if H+ ions and metal ions are present, hydrogen gas will be produced if the metal ions are more reactive than H+ ions (for example, sodium ions). If the metal ions are less reactive than the H+ ions (for example, copper ions), a solid layer of the pure metal will be produced.
At the anode, if OH- and halide ions (Cl-, Br-, I-) are present, then molecules of chlorine, bromine or iodine will be formed. If no halide ions are present, then oxygen gas and water will be formed.
Electrolysis of sulphuric acid
A solution of sulphuric acid (H2SO4) contains three different ions: SO42− , H+ and OH-.
At the cathode: as sulphur (SO42−) is more reactive than hydrogen, hydrogen gas is produced...
2H+ + 2e- ---> H2
At the anode: as there no halide ions present, oxygen and water is produced.
4OH- ---> O2 + 2H2O + 4e-
Electrolysis of sodium chloride
A solution of sodium chloride (NaCl) contains four different ions: Na+, Cl-, OH- and H+
At the cathode: as sodium is more reactive than hydrogen, hydrogen gas is produced...
2H+ + 2e- ---> H2
At the anode: as chlorine ions are present (halide), then chlorine atoms will be produced (as chlorine gas)...
2Cl- ---> Cl2 + 2e-
Electrolysis of copper(II) sulfate
A solution of copper(II) sulphate (CuSO4) contains four different ions: Cu2+, SO42−, H+ and OH-.
At the cathode: as copper is less reactive than hydrogen, copper metal is produced...
Cu2+ + 2e- ---> Cu
At the anode: as there are no halide ions present, oxygen and water are produced...
4OH- ---> O2 + 2H2O + 4e-
Products
At the cathode, if H+ ions and metal ions are present, hydrogen gas will be produced if the metal ions are more reactive than H+ ions (for example, sodium ions). If the metal ions are less reactive than the H+ ions (for example, copper ions), a solid layer of the pure metal will be produced.
At the anode, if OH- and halide ions (Cl-, Br-, I-) are present, then molecules of chlorine, bromine or iodine will be formed. If no halide ions are present, then oxygen gas and water will be formed.
Electrolysis of sulphuric acid
A solution of sulphuric acid (H2SO4) contains three different ions: SO42− , H+ and OH-.
At the cathode: as sulphur (SO42−) is more reactive than hydrogen, hydrogen gas is produced...
2H+ + 2e- ---> H2
At the anode: as there no halide ions present, oxygen and water is produced.
4OH- ---> O2 + 2H2O + 4e-
Electrolysis of sodium chloride
A solution of sodium chloride (NaCl) contains four different ions: Na+, Cl-, OH- and H+
At the cathode: as sodium is more reactive than hydrogen, hydrogen gas is produced...
2H+ + 2e- ---> H2
At the anode: as chlorine ions are present (halide), then chlorine atoms will be produced (as chlorine gas)...
2Cl- ---> Cl2 + 2e-
Electrolysis of copper(II) sulfate
A solution of copper(II) sulphate (CuSO4) contains four different ions: Cu2+, SO42−, H+ and OH-.
At the cathode: as copper is less reactive than hydrogen, copper metal is produced...
Cu2+ + 2e- ---> Cu
At the anode: as there are no halide ions present, oxygen and water are produced...
4OH- ---> O2 + 2H2O + 4e-
1.53 describe experiments to investigate electrolysis, using inert electrodes, of molten salts such as lead(II) bromide and predict the products.
Firstly, inert electrodes are just ones that don't react easily (like at all).
NOTE: The cathode (negative) attracts Pb2+ ions as they are positive, the anode (positive) attracts Br- ions as they are negative
 |
NOTE: The cathode (negative) attracts Pb2+ ions as they are positive, the anode (positive) attracts Br- ions as they are negative
- As soon as the lead(II) Bromide melts(becomes molten), the ions become free to move around, this movement enables the ions move allowing a charge to flow, meaning electrolysis can take place.
- The electrodes are made out of carbon - which is inert (unreactive).
- Connect the electrodes to a power source
- The positive lead (II) ions are attracted to the cathode, which is the negative electrode. When they get there, they gain 2 electrons each from the electrode. This forms lead atoms (they are no longer ions as they have no charge). These 'fall' to the bottom of the container as molten lead.
- Bromide ions (negative) are attracted to the positive anode. When they get there, the extra electron which makes the bromide ion negatively charged moves onto the anode, this loss of the extra electron turns each bromide ion into a bromine atom. These join in pairs (bond covalently) to form bromine molecules (which is gas).
The half equations...
At the cathode: Pb2+ + 2e- ----> Pb
At the anode: 2Br- ---> Br2 + 2e-
1.52 understand that electrolysis involves the formation of new substances when ionic compounds conduct electricity
During electrolysis, ionic compounds conduct electricity (because they are molten so they have free ions). During electrolysis, negative ions (anions) are attracted to the positive electrode (the anode) and positive ions (cations) are attracted to the negative electrode (the cathode). For the circuit to be complete there has to be a flow of electrons. For this to occur, electrons are taken away from negative ions at the anode (positive electrode) and given to positive ions at the cathode (negative electrode). This means that the ions in the electrolyte undergo a reaction whereby they either gain or lose electrons (reduction or oxidation) and so a new product is formed at each electrode (because as ions lose electrons, they become atoms or molecules).
1.51 describe experiments to distinguish between electrolytes and non-electrolytes
When you place a conductivity probe in an electrolyte, current will flow through the circuit - this means you can measure its conductivity.
When you place conductivity probe in a non-electrolyte, no current will flow - this means you will get a reading of 0 conductivity
Another way to determine whether a substance is an electrolyte or non-electrolyte is to set up an electrolytic cell - if the substance will undergo electrolysis then it is an electrolyte, if not, it is not.
When you place conductivity probe in a non-electrolyte, no current will flow - this means you will get a reading of 0 conductivity
Another way to determine whether a substance is an electrolyte or non-electrolyte is to set up an electrolytic cell - if the substance will undergo electrolysis then it is an electrolyte, if not, it is not.
1.50 understand why ionic compound conduct electricity only when molten or in solution
Electrolysis requires an electrolyte (a liquid that an conduct electricity), electrolytes are made by melting or dissolving ionic compounds, this results in free ions which conduct electricity. Molten ionic compounds can conduct electricity because the ions can move freely.
1.49 understand why covalent compounds do not conduct electricity
In a covalent compound, there are no delocalised electrons, so it cannot hold a current (in other words, there are no electrons free to move, therefore there can be no transfer of electricity).
NOTE: Graphite is an exception as this is a giant covalent structure and has the 4th ion free to move, so graphite can conduct electricity
1.48 understand that an electric current is a flow of electrons or ions
An electric current is a flow of electrons, it an also be a flow of ions (as the are charged). Its basically a flow of charged particles.
1.47 explain the electrical conductivity and malleability of a metal in terms of its structure and bonding.
Metals are a giant 3-D structure of positive ions surrounded by delocalized electrons.
Electrons carry electricity. Metals are good conductors of electricity as they have lots of delocalized ('free') electrons that are free to move when a voltage is applied, carrying a charge through the metal.
Metals are structured with layers of positive ions on top of eachother. These ions can easily slide over one another as (in pure metals) they will all be the same size. As they can easily slide, this means they are malleable and ductile.
NOTE: The metallic bonds (force between cations and delocalized electrons) are not broken when metals are moulded as the electrons 'travel' with the cations.
Electrons carry electricity. Metals are good conductors of electricity as they have lots of delocalized ('free') electrons that are free to move when a voltage is applied, carrying a charge through the metal.
Metals are structured with layers of positive ions on top of eachother. These ions can easily slide over one another as (in pure metals) they will all be the same size. As they can easily slide, this means they are malleable and ductile.
NOTE: The metallic bonds (force between cations and delocalized electrons) are not broken when metals are moulded as the electrons 'travel' with the cations.
1.45 explain how the uses of diamond and graphite depend on their structures, limited to graphite as a lubricant and diamond in cutting
Diamond
In diamond, each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. As a result, diamond is very hard and has a high melting point. This explains why it is used in cutting tools
Graphite
In graphite, each carbon atom is joined to only three other carbon atoms, this results in carbon sheets that are 'stacked' on top of each other. These layers can slide over each other, this means that graphite is much softer than diamond. It is used in pencils, and as a lubricant
NOTE: Diamond does not conduct electricity but graphite does
In diamond, each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. As a result, diamond is very hard and has a high melting point. This explains why it is used in cutting tools
Graphite
In graphite, each carbon atom is joined to only three other carbon atoms, this results in carbon sheets that are 'stacked' on top of each other. These layers can slide over each other, this means that graphite is much softer than diamond. It is used in pencils, and as a lubricant
NOTE: Diamond does not conduct electricity but graphite does
1.43 explain the high melting and boiling points of substances with giant covalent structures in terms of the breaking of many strong covalent bonds
Giant covalent structures are very like giant ionic structures only they do not have charged ions. Instead, all atoms are bonded to each other by strong covalent bonds. There are a lot of these bonds which means it takes a lot of energy to break them, therefore they have very high melting and boiling points
1.42 explain why substances with simple molecular structure have low melting and boiling points in terms of the relatively weak forces between the molecules
The atoms within a molecule are held together with strong covalent bonds. In contrast, the intermolecular forces between molecules are very weak and as a result the boiling and melting points of simple molecular substances are very low (because the molecules are easily parted from each other)
1.41 understand that substances with simple molecular structures are gases or liquids, or solids wit low melting points
You can tell when a substance has a simple molecular structure from its physical state (at room temperature). Most molecular substances are liquid or gas (at room temperature) but some are solid with a low melting point
1.40 explain, using dot and cross diagrams, the formation of covalent compounds by electron sharing for the following substances...
For each of the diagrams I have added a little explanation of what's happening to aid understanding, if you know what happens ignore the writing, otherwise read it as it may be useful :)
Hydrogen (H2)
Hydrogen atoms only have 1 electron and they only need 1 more to compete their shell (remember: the first shell only needs two electrons to be complete). To complete their shell, they form a covalent bond. Only one pair of electrons is shared between them, this molecule is known as H2 (hydrogen gas)

Chlorine (Cl2)
Much like hydrogen, chlorine atoms also only need one more electron...

This is Cl2 (as there are 2 Cl atoms in the molecule)
Hydrogen chloride (HCl)
As hydrogen and chlorine both only need one electron to complete their outer shell, they can bond with each other, this forms HCl...

Water (H2O)
Oxygen atoms have 6 electrons on their outer shell and therefore need 2 more electrons to complete their shell. However, hydrogen only needs one. This means that one oxygen atom must bond with two hydrogen atoms...

Methane (CH4)
Carbon has 4 outer electrons, therefore it needs 4 more to complete its outer electron shell. However, hydrogen only needs 1 more electron. This means that one carbon atom bonds with four hydrogen atoms

Ammonia (NH3)
Nitrogen has 5 electrons on its outer shell, so needs 3 more electrons to compete its shell, but hydrogen only needs 1 more electron. This means that 1 nitrogen atom bonds with 3 hydrogen atoms...

Oxygen (O2)
In oxygen gas one oxygen atom shares TWO pairs of electrons with another oxygen atom to complete its shell, this is known as a double covalent bond

Nitrogen (N2)
Nitrogen atoms have 5 electrons on their outer shell, therefore they need 3 more electrons to complete their shell. This means that two nitrogen atoms share THREE pairs of electrons to fill their outer shells, this is known as a triple covalent bond

Carbon dioxide (CO2)
In carbon dioxide. two oxygen atoms share two pairs of electrons with a carbon atom, this forms two double covalent bonds
Ethane (C2H6)
In ethane there are two carbon atoms and six hydrogen atoms. Each of the six hydrogen atoms share their only electron with one of the two carbon atoms (each carbon atom bonds with 3 hydrogen atoms), the two carbon atoms then share their last electron with each other.
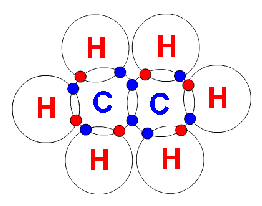
Ethene (C2H4)
In there there are two carbon atoms and four hydrogen atoms. The four hydrogen atoms each share their only electron with one of two carbon atoms, the two carbon atoms then share their lat two electrons with each other, forming a carbon-carbon double covalent bond.
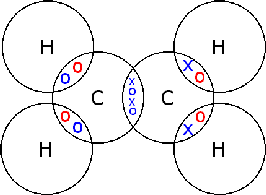
Hydrogen (H2)
Hydrogen atoms only have 1 electron and they only need 1 more to compete their shell (remember: the first shell only needs two electrons to be complete). To complete their shell, they form a covalent bond. Only one pair of electrons is shared between them, this molecule is known as H2 (hydrogen gas)

Chlorine (Cl2)
Much like hydrogen, chlorine atoms also only need one more electron...

This is Cl2 (as there are 2 Cl atoms in the molecule)
Hydrogen chloride (HCl)
As hydrogen and chlorine both only need one electron to complete their outer shell, they can bond with each other, this forms HCl...

Water (H2O)
Oxygen atoms have 6 electrons on their outer shell and therefore need 2 more electrons to complete their shell. However, hydrogen only needs one. This means that one oxygen atom must bond with two hydrogen atoms...

Methane (CH4)
Carbon has 4 outer electrons, therefore it needs 4 more to complete its outer electron shell. However, hydrogen only needs 1 more electron. This means that one carbon atom bonds with four hydrogen atoms

Ammonia (NH3)
Nitrogen has 5 electrons on its outer shell, so needs 3 more electrons to compete its shell, but hydrogen only needs 1 more electron. This means that 1 nitrogen atom bonds with 3 hydrogen atoms...

Oxygen (O2)
In oxygen gas one oxygen atom shares TWO pairs of electrons with another oxygen atom to complete its shell, this is known as a double covalent bond

Nitrogen (N2)
Nitrogen atoms have 5 electrons on their outer shell, therefore they need 3 more electrons to complete their shell. This means that two nitrogen atoms share THREE pairs of electrons to fill their outer shells, this is known as a triple covalent bond

Carbon dioxide (CO2)
In carbon dioxide. two oxygen atoms share two pairs of electrons with a carbon atom, this forms two double covalent bonds
Ethane (C2H6)
In ethane there are two carbon atoms and six hydrogen atoms. Each of the six hydrogen atoms share their only electron with one of the two carbon atoms (each carbon atom bonds with 3 hydrogen atoms), the two carbon atoms then share their last electron with each other.

Ethene (C2H4)
In there there are two carbon atoms and four hydrogen atoms. The four hydrogen atoms each share their only electron with one of two carbon atoms, the two carbon atoms then share their lat two electrons with each other, forming a carbon-carbon double covalent bond.

1.39 understand covalent bonding as a strong attraction between the bonding pair of electrons and the nuclei of the atoms involved in the bond
sorry not much to say here... you just need to learn that in covalent bonding there is a strong attraction between the shared electrons (the 'bonding pair') and the nuclei of the atoms involved
1.38 describe the formation of a covalent bond by sharing a pair of electrons between the two atoms
Covalent bonding is where atoms share electrons with each other so that they have a full outer shell (as aposed to ionic bonding where an electron is 'given away' to another atom)
1.37 draw a diagram to represent the positions of the ions in a crystal of sodium chloride
Sodium chloride has a typical ionic structure, it has alternating positive (Na+) and negative (Cl-) ions, this is how it should be drawn...


Subscribe to:
Posts (Atom)


