In the presence if UV light, halogens will react with alkanes (producing haloalkanes). When this reaction occurs, a hydrocarbon atom from the alkane is replaced by the halogen (chlorine or bromine). This is known as a substitution reaction...

methane + bromine ---> bromomethane + Hydrogen bromine
NOTE: The reaction must have UV light to work, just like photosynthesis can't work without light, substitution reactions will not work without UV light.
If there is lots of oxygen, complete combustion will occur. This releases lots of energy whilst producing water and carbon dioxide. These are the equations for the combustion of methane, for example...
methane + oxygen ---> carbon dioxide + water
CH4 + 2O2 → CO2 + 2H2O
NOTE: When there is lots of oxygen and the combustion is complete, the gas burns with a clean blue flame.
Incomplete combustion occurs when there is not enough oxygen. Less energy is r=produced than in complete combustion, along with this energy, water and carbon dioxide, carbon monoxide is also produced(which is poisonous). These are the equations for an example of incomplete combustion...
4CH4 + 6O2 → C + 2CO + CO2 + 8H2O
NOTE: Incomplete combustion will burn with a smoky yellow flame. Also, the product will depend on how much oxygen is present (e.g, using the example above, is 7O2 were present, no C would be produced, instead, it would be another CO2).
Alkanes have the general formula CnH2n+2 . The best way to think of this is that each carbon is bonded with two hydrogen atoms, then there's two on each end...
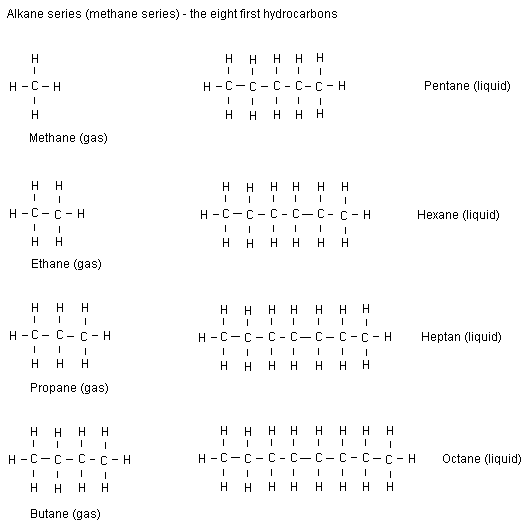 NOTE: we only need to learn up to pentane.
NOTE: we only need to learn up to pentane.
Not much to explain here... alkanes have the general formula CnH2n+2
