Halide ions
To test for halide ions, add dilute nitric acid (HNO3) followed by silver nitrate solution (AgNO3). A precipitate will be produces, the colour of this precipitate will determine what ions are present.
Cl - > white precipitate
Br- > cream precipitate
I- > yellow precipitate
NOTE: The silver nitrate solution determines which halide ions are present. The dilute nitric acid is added to 'get rid' of an carbonate or sulphite ions (as these would react with the silver nitrate).
Sulphate ions
To test for sulphate ions (SO42- ), add dilute HCl and then barium chloride solution (BaCl2). If sulphate ions are present, a note precipitate would form (this precipitate is barium sulphate)
NOTE: The HCl is used to 'get rid' of any carbonate ions, and these may impede results if present as they would also produce a precipitate).
Carbonate ions
To test for carbonate ions (CO32- ), add dilute HCl to the sample you are testing. If a gas is produced, collect it and bubble it through limewater. If carbonate ions are present, the limewater will go cloudy as carbon dioxide will be released.
A blog covering and explaining the Edexcel IGCSE Chemistry specification for the 2016 summer exams. If you are doing just double science, you do not need to learn the stuff for paper two, if you are doing triple you will need to learn all (GOOD LUCK!) I have separated the papers to make files easier to find. Hope it helps :)
Wednesday, 18 May 2016
1.55 write ionic half equations representing the reactions at the electrodes during electrolysis
Half equations just show what happened at each electrode. Like any equation, they need to be balanced. here are some examples...
2Br- > Br2 + 2e-
Above is an equation of a reaction at an anode. We can tell this because the Br2 loses an electron. This can be inferred as on the left it is 2Br- and on the right it is Br2 (meaning it has lost an electrode as it no longer is a negative ion, it is an atom). As it is 2Br-, 2 electrons must be lost.
2H+ + 2e- > H2
Above is an equation of a reaction at a cathode as 2H+ gains 2 electrons to become H2. The charges cancel out and it is neutral on both sides. As there are 2 'H's, there must be 2 electrons gained.
2Br- > Br2 + 2e-
Above is an equation of a reaction at an anode. We can tell this because the Br2 loses an electron. This can be inferred as on the left it is 2Br- and on the right it is Br2 (meaning it has lost an electrode as it no longer is a negative ion, it is an atom). As it is 2Br-, 2 electrons must be lost.
2H+ + 2e- > H2
Above is an equation of a reaction at a cathode as 2H+ gains 2 electrons to become H2. The charges cancel out and it is neutral on both sides. As there are 2 'H's, there must be 2 electrons gained.
1.46 understand that a metal can be described as a giant structuree of positive ions surrounded by a sea of delocalised electrons
In a metal, the atoms are ionically bonded in a giant 3-D structure. The outer shell electrons become detached (making positive ions, cations). The cations are 'floating' in a sea of delocalised electrons. The metal structure stays together because the cations (+) are attracted to the electrons (-). This is known as a metallic bond.
1.31 deduce the charge of an ion from the electronic configuration of the atom from which the ion is formed
An atom with less then four electrons on its outer shell will want to lose electrons because that is the quickest way for it to have a full outer shell. So we know the atom will lose electrons (this makes positive ions).
Atoms with more than four electrons will gain electrons to fill their outer shell (as it is easier than losing electrons). This will result in negative ions.
Here are some examples...
Na has the electronic configuration of 2.8.1 (it has 1 electron on it's outer shell, so it is easiest to lose 1 electron to make a full outer shell). This results in the positive ion (a cation), Na+, which has the electronic configuration of 2.8
Cl has the electronic configuration of 2.8.7 (it has 7 electrons on it's outer shell, so it is easiest to gain 1 electron to make a full outer shell). This results in the negative ion (an anion), Cl-, which has the electronic configuration of 2.8.8
Cl has the electronic configuration of 2.8.7 (it has 7 electrons on it's outer shell, so it is easiest to gain 1 electron to make a full outer shell). This results in the negative ion (an anion), Cl-, which has the electronic configuration of 2.8.8
Tuesday, 17 May 2016
5.22 understand that nitrogen from air, and ydrogen from natural gas or the cracking of hydrocarbons, are used in the manufacture of ammonia
Ammonia is manufactured using hydrogen and nitrogen (as ammonia is NH3). The equation for the reaction is...
N2 (g) + 3H2 (g) ⇌ + 2NH3 (g) (+heat)
Hydrogen is sourced from natural gas. Nitrogen is sourced from the air (as the air is 78% nitrogen).
The manufacture of ammonia with hydrogen and nitrogen is reversible, therefore, as soon as ammonia is made, it starts to turn back into nitrogen and hydrogen. This mean that not all of the nitrogen and hydrogen is converted to ammonia and not all ammonia is converted into nitrogen and hydrogen (because, as soon as nitrogen and hydrogen are produced, it starts to turn into ammonia). This means the reaction reached a dynamic equilibrium.
N2 (g) + 3H2 (g) ⇌ + 2NH3 (g) (+heat)
Hydrogen is sourced from natural gas. Nitrogen is sourced from the air (as the air is 78% nitrogen).
The manufacture of ammonia with hydrogen and nitrogen is reversible, therefore, as soon as ammonia is made, it starts to turn back into nitrogen and hydrogen. This mean that not all of the nitrogen and hydrogen is converted to ammonia and not all ammonia is converted into nitrogen and hydrogen (because, as soon as nitrogen and hydrogen are produced, it starts to turn into ammonia). This means the reaction reached a dynamic equilibrium.
Sunday, 15 May 2016
5.21 understamd that condensation polymerisation produces a small molecule, such as water, as well as the polymer
When condensation polymerisation occurs, a small molecule (such as water) is produces, as well as the monomer.
5.20 understand that some polymers, such as nylon, form by a different process called condensation poymerisation
Most often, condensation polymerisation involves two different types of monomer. These monomers react together forming bonds between them, making polymer chains. However, for each new bond that forms, a molecule (e.g. water) is lost.
5.19 explain that addition polymers are hard to dispose of as their inertness means that they do not easily biodegrade
Most addition polymers are inert. All this means is that they do not react easily (due to the fact that the carbon bonds are very strong and hard to break). This means that it takes a really long time for addition polymers to biodegrade. Furthermore, burning plastics releases toxic chemicals so that's not a good idea either. Because of this, it is hard to dispose of polymers (that's why we recycle them).
5.18 describe some uses for polymers, including poly(ethene), poly(propene) and poly(chloroethene)
Polyethene is light and stretchy making it ideal for packaging such as plastic bags, water bottles/food containers and carpets.
Polypropene is a tough polymer but is quite flexible and heat resistant too, this makes it ideal for making things like crates
Polychloroethene is used to make clothes, drainpipes and insulating cables
Polypropene is a tough polymer but is quite flexible and heat resistant too, this makes it ideal for making things like crates
Polychloroethene is used to make clothes, drainpipes and insulating cables
5.17 deduce the structure of a monomer from the repeat unit of an addition polymer
To find the structure of a monomer, take the repeat unit of an addition polymer and add a double bond between the two carbons.
5.16 draw the repeat unit of addition polymers, including poly(ethene), poly(propene) and poly(chloroethene)
5.15 understand that an addition polymer is formed by joining up many small molecules called monomers
A polymer is just a very long saturated chain (saturated as it has no carbon double bonds). Small molecules called monomers join together to make polymers. One type of polymer is an addition polymer. The monomers that make up addition polymers are alkenes as they have a carbon-carbon double bond. If alkenes are put under a high pressure with a catalyst, they will break their carbon-carbon double bond and polymerise (join together), forming a polymer.
5.14 describe how lng-chain alkanes are converted to alkenes and shorter-chaiin alkanes by catalytic cracking, using silica or alumina as the atalyst and a temperature in the range of 600-700°C
If carrying out the reaction in a lab...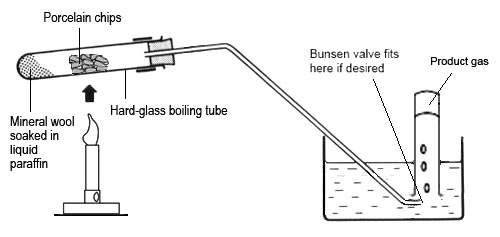
1- Heat the hydrocarbon (e.g. paraffin).
2- After a few seconds, move the Bunsen burner to heat the silica/aluminium catalyst
3- Alternate between the two until the paraffin vaporises and the catalyst glows red
4- The heated paraffin vapour cracks as it passes over the heated catalyst
5- small alkanes collect at the end of the boiling tube, while alkene gases travel down the delivery tube
6- The alkenes are collected through water using a glass jar
In industry, vapourised hydrocarbons are passed over a powdered catalyst at about 600-700°C. silica and aluminium are used as catalysts.
Notes credit: CGP

1- Heat the hydrocarbon (e.g. paraffin).
2- After a few seconds, move the Bunsen burner to heat the silica/aluminium catalyst
3- Alternate between the two until the paraffin vaporises and the catalyst glows red
4- The heated paraffin vapour cracks as it passes over the heated catalyst
5- small alkanes collect at the end of the boiling tube, while alkene gases travel down the delivery tube
6- The alkenes are collected through water using a glass jar
In industry, vapourised hydrocarbons are passed over a powdered catalyst at about 600-700°C. silica and aluminium are used as catalysts.
Notes credit: CGP
5.13 understand that fractional distillation of crude oil produces more long-chain hydrocarbons than can be used directly and fewer short-chain hydrocarbons than required and explain why this makes cracking necessary
Fractional distillation produces lots of long chain hydrocarbons and not many short chain hydrocarbons in relative. The short chain hydrocarbons are useful but long chain hydrocarbons are not. This means we have to break the long chain hydrocarbons into short chain hydrocarbons as demand for short chain hydrocarbons is high. This is done in a process known as cracking. This process is a form of thermal decomposition, which just means breaking down the molecules into simpler molecules by heating them.
5.12 understand that nitrogen oxides and sulfur dioxide are pollutant gases which contribute to acid raid, and describe the problems caused by acid rain
Sulfur dioxide mix with clouds forming dilute sulfuric acid, which is very acidic. Nitrous oxide also mixes with clouds, forming nitric acid. These fall as acid rain which causes lakes to become acid in (killing plants and animals), kills trees and damages limestone buildings and statues.
There have also been links made between acid rain and human health although these are not proven.
There have also been links made between acid rain and human health although these are not proven.
Wednesday, 4 May 2016
5.11 understand that, in car engines, the temperature reached is high enough to allow nitrogen and oxygen from air to react, forming nitrogen oxides
In car engines, crude oil fractions (such as diesel or petrol) are burnt (as fuels). When fossil fuels are burnt, nitrogen oxide and sulfur dioxide are always released, always. When the temperature is high enough, the nitrogen and oxygen in the air react (this creates nitrous oxides, such as nitrogen monoxide and nitrogen dioxide).
NOTE: remember nitrogen oxide and sulfur dioxide are always produced when fossil fuels are burnt.
NOTE: remember nitrogen oxide and sulfur dioxide are always produced when fossil fuels are burnt.
5.10 understand that incomplete combustion of fuels may produce carbon monoxide and explain that carbon monoxide is poisonous because it reduces the capacity of the blood from air to react, forming nitrogen oxides
First of all, incomplete combustion just means that the substance/thing was burnt without lots of oxygen present. When hydrocarbon fuels (for example gas or petrol) is burnt without oxygen, carbon monoxide is produced (as apposed to carbon dioxide, with complete combustion). This is bad because carbon monoxide is poisonous. This is because it combines with haemoglobin (this carried oxygen in your blood), preventing as much oxygen to get to your cells (as less oxygen can bind with the haemoglobin as there is lots of carbon monoxide combined with the haemoglobin). Therefore, less oxygen is being carried around your body (in your blood), this can lead to fainting, a coma or even death (in severe circumstances) as your cells are effectively being starved of oxygen.
Sorry for delays!
As I have been back at school I have not been able to keep up with the blog as much as I was over Easter break.. I leave school this Friday for study leave and will be finishing all blogs next week (iGCSE Chemistry, iGCSE Physics and iGCSE Biology), sorry for any inconvenience, thank you for sticking with me! If you have any questions about any posts do not hesitate to comment and I will reply as soon as possibly, hope any exams you have already done went smoothly (for those doing Cambridge iGCSE english... what was the obsession with the bees?), good luck for any doing art and textiles exams over the next few days also. I will endeavour to finish the blogs as soon as possible next week so I have all the rest of study leave to answer any questions or anything you have. Good luck once more, I know you will all do amazing :)
Millie
Millie
Subscribe to:
Posts (Atom)


