If you have ethanol and you want ethene, you can dehydrate it. This is done by removing water from the ethanol and is known as a dehydration reaction.
Method
Ethanol vapour is passed over a hot catalyst (aluminium oxide, Al2O3)
NOTE: The catalyst is used as it provides a large surface area for the reaction to take place, meaning the reaction will be quick(ish)
A blog covering and explaining the Edexcel IGCSE Chemistry specification for the 2016 summer exams. If you are doing just double science, you do not need to learn the stuff for paper two, if you are doing triple you will need to learn all (GOOD LUCK!) I have separated the papers to make files easier to find. Hope it helps :)
Showing posts with label Section 3. Show all posts
Showing posts with label Section 3. Show all posts
Friday, 1 April 2016
3.11 evaluate the factors relevant to the choice of method used in the manufacture of ethanol, for example the relative availability of sugar cane and crude oil
Producing ethanol by reacting ethene and steam is relatively cheap (as, right now, ethane is quite cheap and not much is wasted). However, ethene is produced from crude oil which is a finite source, soon it will become fairly expensive as it gets rarer and in the end it will run out, meaning ethanol will no longer be able to be made using ethene and steam.
An advantage of making ethanol by fermenting sugars is that all 'reactants' are renewable (sugar and yeast). It also can be produced at a much lower temperature. However, the ethanol you get from fermenting sugars is not as concentrated as when you react steam with ethene (basically, its super weak). This needs to be distilled to increase its strength and it also needs to be purified. So, although its simpler, its also a lot more hassle.
An advantage of making ethanol by fermenting sugars is that all 'reactants' are renewable (sugar and yeast). It also can be produced at a much lower temperature. However, the ethanol you get from fermenting sugars is not as concentrated as when you react steam with ethene (basically, its super weak). This needs to be distilled to increase its strength and it also needs to be purified. So, although its simpler, its also a lot more hassle.
3.10 describe the manufacture of ethanol by the fermentation of sugars, for example glucose, at a temperature of about 30ºC
Another method of producing ethanol is by fermentation. The raw material for fermentation is sugar (e.g. glucose), which is converted into ethanol using yeast. This process is done at 30ºC.
3.9 describe the manufacture of ethanol by passing ethene and steam over a phosphoric acid catalyst at a temperature of about 300ºC and a pressure of about 60-70 atm
Ethene will react with steam to produce ethanol. This reaction will take place at 300ºC with a pressure of 60-70 atm. This process is very slow, so a catalyst of phosphoric acid is used.
NOTE: atm stands for atmospheres
NOTE: atm stands for atmospheres
3.8 describe the addition of alkenes with bromine, including the decolourising of bromine water as a test for alkenes
Halogens can react with alkenes to form haloalkenes (this does not need UV light, unlike the formation of haloalkanes). For example, bromine ad ethene react together, forming dibromoethane (as it is composed of two bromine atoms and an ethene molecule). This is known as an addition reaction as the carbon-carbon couple bond is split and a halogen atom (in the case, bromine) is added to each carbon.


ethene + bromine ---> dibromoethane
This reaction can also be used to determine whether a substance is an alkene or not. This is because if you add an ethene to bromine, the solution formed (in this case, dibromoethane) will be colourless. If the unknown solution does not contain an alkene, the solution will stay the colour of bromine (yellow-brown).
3.7 draw displayed formulae for alkenes with up to four carbon atoms in a molecule, and name the straight-chain isomers (knowledge of cis- and trans-isomers is not required
This one gets a bit more confusing than alkanes. Alkenes have one carbon-carbon double bond in their carbon chain (this means they are saturated).
There are two possibilities for butene as the carbon-carbon double bond can go in two places.
| alkene | formula | chemical structure | ball-and-stick model |
|---|---|---|---|
| ethene | C2H4 |  |  |
| propene | C3H6 |  |  |
| butene | C4H8 |  |  |
NOTE: Ethene is the first alkene as 'methene' can not exist. This is because alkenes have carbon-carbon double bonds and 'methene' would only have 1 carbon, with no double bond.
3.6 recall that alkenes have the general formula CnH2n
Not much to explain here... alkenes have the general formula CnH2n
3.5 describe the substitution reaction of methane with bromine to form bromomethane in the presence of UV light
In the presence if UV light, halogens will react with alkanes (producing haloalkanes). When this reaction occurs, a hydrocarbon atom from the alkane is replaced by the halogen (chlorine or bromine). This is known as a substitution reaction...

methane + bromine ---> bromomethane + Hydrogen bromine
NOTE: The reaction must have UV light to work, just like photosynthesis can't work without light, substitution reactions will not work without UV light.
methane + bromine ---> bromomethane + Hydrogen bromine
NOTE: The reaction must have UV light to work, just like photosynthesis can't work without light, substitution reactions will not work without UV light.
3.4 recall the products of the complete and incomplete combustion of alkanes
If there is lots of oxygen, complete combustion will occur. This releases lots of energy whilst producing water and carbon dioxide. These are the equations for the combustion of methane, for example...
methane + oxygen ---> carbon dioxide + water
CH4 + 2O2 → CO2 + 2H2O
NOTE: When there is lots of oxygen and the combustion is complete, the gas burns with a clean blue flame.
Incomplete combustion occurs when there is not enough oxygen. Less energy is r=produced than in complete combustion, along with this energy, water and carbon dioxide, carbon monoxide is also produced(which is poisonous). These are the equations for an example of incomplete combustion...
4CH4 + 6O2 → C + 2CO + CO2 + 8H2O
NOTE: Incomplete combustion will burn with a smoky yellow flame. Also, the product will depend on how much oxygen is present (e.g, using the example above, is 7O2 were present, no C would be produced, instead, it would be another CO2).
methane + oxygen ---> carbon dioxide + water
CH4 + 2O2 → CO2 + 2H2O
NOTE: When there is lots of oxygen and the combustion is complete, the gas burns with a clean blue flame.
Incomplete combustion occurs when there is not enough oxygen. Less energy is r=produced than in complete combustion, along with this energy, water and carbon dioxide, carbon monoxide is also produced(which is poisonous). These are the equations for an example of incomplete combustion...
4CH4 + 6O2 → C + 2CO + CO2 + 8H2O
NOTE: Incomplete combustion will burn with a smoky yellow flame. Also, the product will depend on how much oxygen is present (e.g, using the example above, is 7O2 were present, no C would be produced, instead, it would be another CO2).
3.3 draw displayed formula for alkanes with up to five carbon atoms in a molecule, and name the straight chain isomers
Alkanes have the general formula CnH2n+2 . The best way to think of this is that each carbon is bonded with two hydrogen atoms, then there's two on each end...
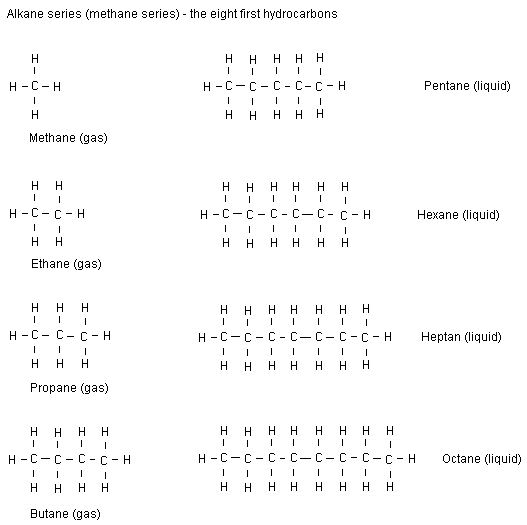
NOTE: we only need to learn up to pentane.

NOTE: we only need to learn up to pentane.
3.2 recall that alkanes have the general formula CnH2n+2
Not much to explain here... alkanes have the general formula CnH2n+2
3.1 explain the terms homologous series, hydrocarbon, saturated, unsaturated, general formula and isomerism
Homologous series
A group of compound that can all be represented by the same general formula
Hydrocarbon
Molecules made up of hydrogen and carbon atoms only
Saturated
A molecule that contains only single bonds
Unsaturated
A molecule that contains carbon-carbon double bonds (NOTE: must say carbon-carbon for the mark)
General formula
A formula that allows us to work out the number of carbon and hydrogen atoms in hydrocarbons (for example)
Isomerism
Compounds with the same molecular formula but a different display formula
A group of compound that can all be represented by the same general formula
Hydrocarbon
Molecules made up of hydrogen and carbon atoms only
Saturated
A molecule that contains only single bonds
Unsaturated
A molecule that contains carbon-carbon double bonds (NOTE: must say carbon-carbon for the mark)
General formula
A formula that allows us to work out the number of carbon and hydrogen atoms in hydrocarbons (for example)
Isomerism
Compounds with the same molecular formula but a different display formula
Subscribe to:
Posts (Atom)