Hydrogen
Squeaky pop test - put a lit splint into a test tube of gas. If it makes a 'squeaky pop' sound, hydrogen is in the tube.
Oxygen
Oxygen will relight glowing splint.
Carbon Dioxide
If bubbled through limewater the limewater will go cloudy if carbon dioxide is present.
Ammonia
Damp red litmus paper will turn blue if ammonia is present.
Chlorine
Turns damp blue litmus paper red, then white (as it bleaches).
A blog covering and explaining the Edexcel IGCSE Chemistry specification for the 2016 summer exams. If you are doing just double science, you do not need to learn the stuff for paper two, if you are doing triple you will need to learn all (GOOD LUCK!) I have separated the papers to make files easier to find. Hope it helps :)
Thursday, 31 March 2016
2.37 describe the tests for the cations...
Flame tests
To do a flame test, dip a platinum wire loop in dilute HCL then hold it in a flame, take it out once the flame burns without a colour (this means the platinum is clean). Dip the loop in the sample your testing and put it back in the flame, the colour of the flame will tell you what metal ion is in the substance...
Li+ > burns with a crimson red flame
Na+ > burns with a yellow-orange flame
K+ > burns with a lilac flame
Ca2+ > burns with a brick red flame
Sodium hydroxide solution and identify the ammonia evolved (NH4+)
You can check for ammonia gas using damp red litmus paper (will turn from red to blue is ammonia is present).
To test for ammonium ions in an unknown substance, add a few drops of sodium hydroxide solution to a solution of the unknown substance (in a test tube). Hold a piece of litmus paper near the top of the test tube, if ammonia is being given off, ammonium ions are present in the unknown substance.
Sodium hydroxide
Metal hydroxides are insoluble and precipitate out of solution when formed.
For this test add a few drops of sodium hydroxide solution to a solution the 'mystery compound' (what your testing). This will form an insoluble hydroxide. Some of these hydroxides have characteristic colours, this can be used to tell which metal ions are present in the unknown solution...
Cu2+ > blue
Fe2+ > sluggish green (yes, they are genuinely the words used)
Fe3+ > reddish brown
To do a flame test, dip a platinum wire loop in dilute HCL then hold it in a flame, take it out once the flame burns without a colour (this means the platinum is clean). Dip the loop in the sample your testing and put it back in the flame, the colour of the flame will tell you what metal ion is in the substance...
Li+ > burns with a crimson red flame
Na+ > burns with a yellow-orange flame
K+ > burns with a lilac flame
Ca2+ > burns with a brick red flame
Sodium hydroxide solution and identify the ammonia evolved (NH4+)
You can check for ammonia gas using damp red litmus paper (will turn from red to blue is ammonia is present).
To test for ammonium ions in an unknown substance, add a few drops of sodium hydroxide solution to a solution of the unknown substance (in a test tube). Hold a piece of litmus paper near the top of the test tube, if ammonia is being given off, ammonium ions are present in the unknown substance.
Sodium hydroxide
Metal hydroxides are insoluble and precipitate out of solution when formed.
For this test add a few drops of sodium hydroxide solution to a solution the 'mystery compound' (what your testing). This will form an insoluble hydroxide. Some of these hydroxides have characteristic colours, this can be used to tell which metal ions are present in the unknown solution...
Cu2+ > blue
Fe2+ > sluggish green (yes, they are genuinely the words used)
Fe3+ > reddish brown
2.36 understand the sacrificial protection of iron in terms of the reactivity series
The sacrificial method involves placing a more reactive metal (such as zinc) with the iron. Water and oxygen then react with the sacrificial metal rather than the iron (as its more reactive than the iron).
Zinc is often used as it is more reactive than iron, so zinc will be oxidised instead of iron. A coating of zinc could be sprayed onto the iron object (galvanising). Another method is to bolt big blocks of zinc onto the iron (e.g. a ships hull of under groups iron pipes).
Zinc is often used as it is more reactive than iron, so zinc will be oxidised instead of iron. A coating of zinc could be sprayed onto the iron object (galvanising). Another method is to bolt big blocks of zinc onto the iron (e.g. a ships hull of under groups iron pipes).
2.35 describe how the rusting of irony be prevented by grease, oil, paint, plastic and galvanising
These all create a 'barrier' around the iron, stopping water and/or oxygen from reaching it.
Paint/plastic can also be decorative and can be used on big or small structures (its versatile) and can be decorative (:
Grease/oil can only be used on moving parts e.g. bike chains
Paint/plastic can also be decorative and can be used on big or small structures (its versatile) and can be decorative (:
Grease/oil can only be used on moving parts e.g. bike chains
2.34 describe the conditions under which iron rusts
Rusting ONLY occurs when iron is in contact with both water and oxygen (from the air). The chemical reaction that is taking place is oxidation of iron to form iron(III) oxide (oxidation reaction), water then bonds to the iron(III) oxide and forms hydrated iron(III) oxide - this is rust.
NOTE: I'm not sure if we need the word equation so here it is just incase...
iron + oxygen ---> iron(III) oxide
iron(III) oxide + water ---> hydrated iron(III) oxide
2.33 understand the terms redox, oxidising agent, reducing agent
The oxidising agent is the element that gets reduced, the reducing agent is the element that gets oxidised. Reactions where oxidation and reduction take place are known as 'redox reactions' (REDuctionOXidation)
2.30 describe how reactions with water and dilute acids can be used to deduce the following order of reactivity: potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper
Metals high up in the series (potassium, sodium, lithium and calcium) react very vigorous with water.
Metals in the middle (magnesium, zinc and iron) react with steam but don't react with cold water.
Copper won't react with either steam or water.
The more reactive the element is with water and dilute acid, the further up the series the element is positioned.
Metals in the middle (magnesium, zinc and iron) react with steam but don't react with cold water.
Copper won't react with either steam or water.
The more reactive the element is with water and dilute acid, the further up the series the element is positioned.
Wednesday, 30 March 2016
2.29 understand that metals can be arranged in a reactivity series based on the reactions of the metals and their compounds: potassium, sodium, lithium, calcium, magnesium, aluminium, zinc, iron, copper, silver and gold
The 'reactivity series' lists metals in order of their reactivity with other substances. The most reactive is at the top, while the least reactive is at the bottom. This is the order...
Potassium
Sodium
Lithium
Calcium
Magnesium
Aluminium
Zinc
Iron
Copper
Silver
Gold
KEY:
Super reactive
Fairly reactive
A little bit reactive
Not really at all reactive (basically inert)
NOTE: we need to learn this :( The best way is with an mnemonic. I use Peter.Says.Lions.Cats.Mice.And.Zebras.In.Cages.Suffer.Greatly but if you have another please comment!
2.28 describe a physical test to show whether water is pure
If the substance you are testing boils at 100ºC and/or freezes at 0ºC, it is pure water
2.27 describe the use of anhydrous copper(II) sulphate in the chemical test for water
Anhydrous copper(II) sulphate will turn from white to blue if water is present.
To test for water, all you need to do is add anhydrous copper(II) sulphate, which is a white powder, to the substance you are testing. If the anhydrous copper(II) sulphate turns from white to blue, water is present.
This is a reversible reaction, meaning if you heat the blue powder, it will turn white again. This is because the water has 'left' (it's evaporated)
NOTE: anhydrous means without water, hydrated means with. Therefore, if water is present, anhydrous copper(II) sulphate will turn to hydrated copper(II) sulphate.
NOTE NOTE: this does not show pure water, just that the substance contains water molecules
To test for water, all you need to do is add anhydrous copper(II) sulphate, which is a white powder, to the substance you are testing. If the anhydrous copper(II) sulphate turns from white to blue, water is present.
This is a reversible reaction, meaning if you heat the blue powder, it will turn white again. This is because the water has 'left' (it's evaporated)
NOTE: anhydrous means without water, hydrated means with. Therefore, if water is present, anhydrous copper(II) sulphate will turn to hydrated copper(II) sulphate.
NOTE NOTE: this does not show pure water, just that the substance contains water molecules
2.26 describe the combustion of hydrogen
If hydrogen is burnt in air, water is produced (initially as water vapour, as it is hot, but later condenses to water if cooled)
The equation for this reaction is 2H2+ O2 → 2H2O
The equation for this reaction is 2H2+ O2 → 2H2O
2.25 describe the reactions of dilute hydrochloric acid and dilute sulphuric acids with magnesium, aluminium, zinc and iron
Acid + metal ---> salt + hydrogen
Magnesium
- Reacts vigorously with cold dilute acids
- Produces lots of bubbles
Aluminium
- Little reaction with cold dilute acids (as it has a protective aluminium oxide layer)
- Reacts vigorously with warm dilute acids and produces lots of bubbles
Zinc
- React slowly with dilute acids but more strongly if you heat them up
Iron
Same reactions as zinc.
Magnesium
- Reacts vigorously with cold dilute acids
- Produces lots of bubbles
Aluminium
- Little reaction with cold dilute acids (as it has a protective aluminium oxide layer)
- Reacts vigorously with warm dilute acids and produces lots of bubbles
Zinc
- React slowly with dilute acids but more strongly if you heat them up
Iron
Same reactions as zinc.
NOTE: The more reactive the metal, the faster the reaction. For example, very creative metals (such as sodium) will react explosively, as the reaction is very quick. The speed of reaction is given off by the amount of bubbles that are given off.
2.24 understand the carbon dioxide is a greenhouse gas and may contribute to climate change
Gases in the atmosphere (such as carbon dioxide, methane, water vapour) act as natural insulators, trapping heat from the sun inside Earths atmosphere. This is because they absorb most of the heat that would normally be mediated out into space. They reradiate this trapped heat in all directions, including back towards Earth.
The level of carbon dioxide can increase due to many factors, some as a result of humans. For example, deforestation (fewer CO2 is removed by photosynthesis) and burning fossil fuels (CO2 that was 'locked' in these fuels is being released).
Although the Earth's temperature naturally varies (interglacial and glacial periods), there is reason to believe that extra CO2 has caused the average temperature to increase.
The level of carbon dioxide can increase due to many factors, some as a result of humans. For example, deforestation (fewer CO2 is removed by photosynthesis) and burning fossil fuels (CO2 that was 'locked' in these fuels is being released).
Although the Earth's temperature naturally varies (interglacial and glacial periods), there is reason to believe that extra CO2 has caused the average temperature to increase.
2.23 explain the uses of carbon dioxide in carbonating drinks and in fire extinguishers, in terms of its solubility and density
The stuff that makes your fizzy drinks fizzy is the carbon dioxide. As CO2 is slightly soluble in water and dissolves in drinks when user high pressure, a slightly acidic solutions forms due to the formation of carbonic acid. It eventually goes flat because, once you open the bottle, the CO2 is escaping (when it is flat, its all gone)
CO2 is more dense than air, this makes it perfect for fire extinguishers. The CO2 will sink onto flames and 'suffocate' them (stop the oxygen getting to the flames). As fire needs oxygen to burn, the fire will go out as no oxygen can get to it.
NOTE: CO2 fire extinguishers are only used when water extinguishers aren't safe. For example, when putting out an electrical fire.
CO2 is more dense than air, this makes it perfect for fire extinguishers. The CO2 will sink onto flames and 'suffocate' them (stop the oxygen getting to the flames). As fire needs oxygen to burn, the fire will go out as no oxygen can get to it.
NOTE: CO2 fire extinguishers are only used when water extinguishers aren't safe. For example, when putting out an electrical fire.
2.22 describe the properties of carbon dioxide, limited to its solubility and density
Carbon dioxide is more dense than air (which is why it can be collected using the downward delivery method). It is also soluble in water at high pressure.
2.21 describe the formation of carbon dioxide from the thermal decomposition of metal carbonates such as copper(II) carbonate
The thermal decomposition (heating) of metal carbonates will produce CO2 as, in thermal decomposition, the substance being heated will break down into simpler substances.
Method
- Put some copper(II) carbonate (its a green powder, if your wondering) into a test tube and insert a bung with a delivery tube at the top
- Clamp the test tube at a 90º angle and insert the delivery tube into another test tube (that is positioned vertically)
- Heat the copper(II) carbonate with a bunsen burner.
NOTE: Because CO2 is denser than air, the downward delivery method can be used.
Equations
Copper(II) carbonate ---> copper oxide + carbon dioxide
CuCO3(g) ---> CuO(s) + CO2(g)
Method
- Put some copper(II) carbonate (its a green powder, if your wondering) into a test tube and insert a bung with a delivery tube at the top
- Clamp the test tube at a 90º angle and insert the delivery tube into another test tube (that is positioned vertically)
- Heat the copper(II) carbonate with a bunsen burner.
NOTE: Because CO2 is denser than air, the downward delivery method can be used.
Equations
Copper(II) carbonate ---> copper oxide + carbon dioxide
CuCO3(g) ---> CuO(s) + CO2(g)
2.20 describe the laboratory preparation of carbon dioxide from calcium carbonate and dilute hydrochloric acid
Dilute HCL will react with calcium carbonate (aka marble chips) to produce calcium chloride, water and carbon dioxide...
Method
- Put marble chips at the bottom of a flask
- Fill the flask with hydrochloric acid, it doesn't have to be full, just covering the marble chips
- immediately attach a bung with a delivery tube into an upturned test tube in water
- the carbon dioxide will collect in this test tube
Equations
hydrochloric acid + calcium carbonate ---> calcium chloride + water + carbon dioxide
2HCL(aq) + CaCO3(s) ---> CaCl2(aq) + H2O(l) + CO2(g)
Method
- Put marble chips at the bottom of a flask
- Fill the flask with hydrochloric acid, it doesn't have to be full, just covering the marble chips
- immediately attach a bung with a delivery tube into an upturned test tube in water
- the carbon dioxide will collect in this test tube
Equations
hydrochloric acid + calcium carbonate ---> calcium chloride + water + carbon dioxide
2HCL(aq) + CaCO3(s) ---> CaCl2(aq) + H2O(l) + CO2(g)
Tuesday, 29 March 2016
2.19 describe the reactions of magnesium, carbon and sulphur with oxygen in air, and the acid-base character of the oxides
When anything is burnt, it reacts with oxygen in the air to form oxides (which can have wither acidic or basic character).
Magnesium
When magnesium burns in air, it produces a bright white flame and a white powder is formed (this is magnesium oxide). Magnesium oxide is slightly alkali when dissolved in water.
The equation for the reaction (burning Mg in air) is 2Mg(s) + O2(g) ---> 2MgO(s)
NOTE: This question often comes up in exams so make sure you know it!
Carbon
Carbon will only burn in air if it is very strongly heated. It burns with a yellowy-orangey flame and produces carbon dioxide (as a gas). Carbon dioxide is slightly acidic when dissolved in water.
The equation for the reaction (burning C in air) is C(s) + O2(g) ---> CO2(g)
Sulfur
Sulphur burns (in air) with a pale blue flame and produces sulfur dioxide which is acidic when dissolved in water.
The equation for this reaction (burning S in air) is C(s) + O2(g) ---> SO2(g)
Magnesium
When magnesium burns in air, it produces a bright white flame and a white powder is formed (this is magnesium oxide). Magnesium oxide is slightly alkali when dissolved in water.
The equation for the reaction (burning Mg in air) is 2Mg(s) + O2(g) ---> 2MgO(s)
NOTE: This question often comes up in exams so make sure you know it!
Carbon
Carbon will only burn in air if it is very strongly heated. It burns with a yellowy-orangey flame and produces carbon dioxide (as a gas). Carbon dioxide is slightly acidic when dissolved in water.
The equation for the reaction (burning C in air) is C(s) + O2(g) ---> CO2(g)
Sulfur
Sulphur burns (in air) with a pale blue flame and produces sulfur dioxide which is acidic when dissolved in water.
The equation for this reaction (burning S in air) is C(s) + O2(g) ---> SO2(g)
2.18 describe the laboratory preparation of oxygen from hydrogen peroxide, using manganese(IV) oxide as a catalyst
Hydrogen peroxide will decompose to form oxygen and water. However, this process is very slow so manganese (IV) oxide is added to speed up the decomposition (this acts as a catalyst). The oxygen produced can be collected in two ways...
1. Over water...
- connect a delivery tube to bubble the gas into an upside-down measuring cylinder in a beaker/bowl full of water.
2. In a gas syringe...
- you can use a gas syringe to collect pretty much any gas... just connect it to the flask that the hydrogen peroxide is decomposing in.
NOTE: the equation for this reaction is 2H2O2 (aq) ---> 2H2O (l) + O2 (g)
1. Over water...
- connect a delivery tube to bubble the gas into an upside-down measuring cylinder in a beaker/bowl full of water.
2. In a gas syringe...
- you can use a gas syringe to collect pretty much any gas... just connect it to the flask that the hydrogen peroxide is decomposing in.
NOTE: the equation for this reaction is 2H2O2 (aq) ---> 2H2O (l) + O2 (g)
2.17 explain how experiments involving the reactions of elements such as copper, iron and phosphorus with air can be used to investigate the percentage by volume of oxygen in air
Copper
- When copper is heated, it reacts with oxygen in the air to make copper(II) oxide - this reaction uses up oxygen
- If you heat an excess of copper in a tube and pass it over two syringes, you can use the markers on the syringes to work out how much oxygen as been used up. Conclusion... if you start with 100cm3 of air, you should end up with around 80cm3 air once the reaction is finished (and cooled). This means that 20% of the air has gone, meaning 20% must be oxygen
Iron or phosphorus
Iron will react with oxygen to produce rust, this means iron will remove oxygen from the air.
Method...
- soak some iron wool in acetic acid (this acid will catalyse the reaction)
- push the iron wool at the bottom of a test tube and invert the tube into a beaker of water
- mark the level of the water in the tube at the beginning
- leave the experiment for a set amount of time (e.g. 1 hour)
- mark the level of water in the tube at the end of the experiment
Conclusion...
Over time, the level of the water will rise in the test tube. This is because the iron reacts with the oxygen in the air, making iron oxide (the water rises as it takes the place the oxygen took up).
To work out the percentage of air that is oxygen, mark the level of the water in the tube at the beginning and end of the experiment, then, fill up the tube to each mark and pour the contents into a measuring cylinder to find out the volume of air at the start and end. Using the difference between the start and end volumes, work out the % that has been used to (should be approx 20%).
Phosphorus - you can do a similar experiment with white phosphorus. White phosphorus smoulders in air to produce phosphorus oxide. Use the same calculation method as with iron.
- When copper is heated, it reacts with oxygen in the air to make copper(II) oxide - this reaction uses up oxygen
- If you heat an excess of copper in a tube and pass it over two syringes, you can use the markers on the syringes to work out how much oxygen as been used up. Conclusion... if you start with 100cm3 of air, you should end up with around 80cm3 air once the reaction is finished (and cooled). This means that 20% of the air has gone, meaning 20% must be oxygen
Iron or phosphorus
Iron will react with oxygen to produce rust, this means iron will remove oxygen from the air.
Method...
- soak some iron wool in acetic acid (this acid will catalyse the reaction)
- push the iron wool at the bottom of a test tube and invert the tube into a beaker of water
- mark the level of the water in the tube at the beginning
- leave the experiment for a set amount of time (e.g. 1 hour)
- mark the level of water in the tube at the end of the experiment
Conclusion...
Over time, the level of the water will rise in the test tube. This is because the iron reacts with the oxygen in the air, making iron oxide (the water rises as it takes the place the oxygen took up).
To work out the percentage of air that is oxygen, mark the level of the water in the tube at the beginning and end of the experiment, then, fill up the tube to each mark and pour the contents into a measuring cylinder to find out the volume of air at the start and end. Using the difference between the start and end volumes, work out the % that has been used to (should be approx 20%).
Phosphorus - you can do a similar experiment with white phosphorus. White phosphorus smoulders in air to produce phosphorus oxide. Use the same calculation method as with iron.
2.16 recall the gases present in air and their approximate percentage by volume
78% nitrogen
21% oxygen
nearly 1% argon
0.04% carbon dioxide
2.15 understand these displacement reaction as redox reactions
A loss of electrons is known as oxidation, and a gain of electrons is known as reduction. Displacement reactions involving halogens involve a transfer of electrons, and reduction and oxidation happen simultaneously. For example, in the example here, chorine is reduced and iodine is oxidised.
'redox' is short for 'reduction and oxidation' and, as displacement reactions involving halogens involve the transfer of electrons, they are redox reactions.
NOTE: a good way to remember which is oxidation and which is reduction with the anagram 'O.I.L.R.I.G' (Oxidation.Is.Loss.Reduction.Is.Gain)
'redox' is short for 'reduction and oxidation' and, as displacement reactions involving halogens involve the transfer of electrons, they are redox reactions.
NOTE: a good way to remember which is oxidation and which is reduction with the anagram 'O.I.L.R.I.G' (Oxidation.Is.Loss.Reduction.Is.Gain)
2.14 describe the experiments to demonstrate that a more reactive halogen will displace a less reactive halogen from a solution of one of its salts
A displacement reaction is basically a reaction where the more reactive element displaces (pushes out) a less reactive element from a compound. For example...
Chlorine is more reactive than iodine (as it is higher up in group 7). Therefore, if you add chlorine water to potassium iodide solution, the chloride will react with the potassium to form potassium chloride (basically, it displaces the iodine). The iodine is displaced from the salt (potassium iodide) and just kind of gets left in the water solution (this turns the solution brown).
Chlorine is more reactive than iodine (as it is higher up in group 7). Therefore, if you add chlorine water to potassium iodide solution, the chloride will react with the potassium to form potassium chloride (basically, it displaces the iodine). The iodine is displaced from the salt (potassium iodide) and just kind of gets left in the water solution (this turns the solution brown).
2.13 describe the relative reactivities of the elements in group 7
The elements of group 7 get less reactive as you go down. This is because, at the top of the group, the incomplete shell is near to the nucleus, so the pull from the positive nucleus is greater. Therefore, as you go down the pull from the nucleus gets weaker, so it is easier to 'get rid' of electrons.
Sunday, 27 March 2016
2.12 explain, in terms of dissociation, why hydrogen chloride is acidic in water but not in methylbenzene
If HCL is dissolved in water, it dissociates (splits up) into H+ and Cl- ions. This solution (hydrochloric acid) is acidic because it contains H+ ions.
However, if HCL is dissolved in methylbenzene, it doesn't dissociate into H+ and CL- ions, therefore no H+ ions are present so the solution is not acidic.
However, if HCL is dissolved in methylbenzene, it doesn't dissociate into H+ and CL- ions, therefore no H+ ions are present so the solution is not acidic.
2.11 understand the difference between hydrogen chlorine gas and hydrochloric acid
When hydrogen chloride gas (gas at room temperature) is dissolved in water the hydrogen chloride molecules split (into H+ ions and Cl- ions). This process is known as dissociation. The solution that is formed is hydrochloric acid, which is acidic as it contains H+ ions.
2.10 make predictions about the properties of other halogens in this group
The further down the group you go, the more reactive the elements become, the higher the boiling point, the darker the element will be and the 'more' solid (as it will have a higher boiling point).
2.9 recall the colours and physical states of the elements at room temperature
Chlorine - green - gas
Bromine - red-brown - liquid
Iodine - dark grey - solid
Bromine - red-brown - liquid
Iodine - dark grey - solid
2.8 explain the relative reactivities of the elements in Group 1 in terms of distance between the outer electrons and the nucleus
All elements in Group 1 have 1 electron in their outer shell.
The elements further down the group are more reactive. This is because as you go down the group the outer shell (with the single electron) gets further away from the nucleus (as there are complete shells inbetween them). This means that, because the electron is further away, it is more easily lost (given away).
The elements further down the group are more reactive. This is because as you go down the group the outer shell (with the single electron) gets further away from the nucleus (as there are complete shells inbetween them). This means that, because the electron is further away, it is more easily lost (given away).
2.7 describe the relative reactivates of the elements in Group 1
Lithium reacts a little, however, as you get further down the group they react more. For example (reactions with water)...
Lithium - lithium moves slowly around the container, fizzing, until it disappears (approx. 30 seconds).
Sodium - sodium moves quickly around the container, fizzing rapidly, until it disappears (approx. 20 seconds).
Potassium - potassium fizzes and reacts vigorously until it disappears (approx. 5 seconds)
Lithium - lithium moves slowly around the container, fizzing, until it disappears (approx. 30 seconds).
Sodium - sodium moves quickly around the container, fizzing rapidly, until it disappears (approx. 20 seconds).
Potassium - potassium fizzes and reacts vigorously until it disappears (approx. 5 seconds)
2.6 describe the reactions of these elements with water ad understand that the reactions provide a basis or their recognition as a family of elements
Elements of the same family react in a similar way all. Group 1 elements (e.g. lithium, sodium, potassium) react in a similar way with water, because of this, we can class them as a family of elements.
Lithium, sodium and potassium all react vigorously with water. The reaction produces 2 products, 1 is a metal hydroxide, this solution is alkaline as, when a metal reacts with oxygen, an alkali solution is produced. The other product is hydrogen (which is why you can see effervescence). The word equation for the reaction of sodium (for example) and water is...
sodium + water ---> sodium hydroxide + hydrogen
2Na (s) + 2H2O(l) ---> 2NaOH(aq) + H2(g)
Lithium, sodium and potassium all react vigorously with water. The reaction produces 2 products, 1 is a metal hydroxide, this solution is alkaline as, when a metal reacts with oxygen, an alkali solution is produced. The other product is hydrogen (which is why you can see effervescence). The word equation for the reaction of sodium (for example) and water is...
sodium + water ---> sodium hydroxide + hydrogen
2Na (s) + 2H2O(l) ---> 2NaOH(aq) + H2(g)
2.5 understand that nobel gases (group 0) are a family of inert gases and explain their lack of reactivity in terms of their electronic configurations
Nobel gases (group 0, the last column in the Periodic Table) are inert, this means they don't react with many things at all. This is because the noble gases have a full outer shell, so they have no need to gain or lose electrons.
2.4 understand why elements in the same group of the Period Table have similar chemical properties
Elements in the same group have the same number of elements on their outer shell and therefore have similar chemical properties.
E.g all elements in group 2 have 2 electrons on their outer shell, so they all want to lose 2 electrons. Similarly, all noble gases (group 0) have a full outer shell so are inert as they don't want to lose any electrons.
E.g all elements in group 2 have 2 electrons on their outer shell, so they all want to lose 2 electrons. Similarly, all noble gases (group 0) have a full outer shell so are inert as they don't want to lose any electrons.
2.3 explain the classification of elements as metals or non-metals on the basis of their electrical conductivity and the acid-base character of their oxides
Elements are classified as metals if they conduct electricity (because they allow a charge to pass through them easily). Metal oxides are basic, meaning they will neutralise acids, if metal oxides dissolve they will form a solution with pH of more that 7.
Elements are classified as non-metals if they are poor conductors of electricity. Non-metals are acidic and, if they dissolve, they will form a solution with a pH of less than 7.
Elements are classified as non-metals if they are poor conductors of electricity. Non-metals are acidic and, if they dissolve, they will form a solution with a pH of less than 7.
2.2 recall the positions of metals and non-metals in the periodic table
Kind of imagine a staggered line across the table. Any element left of the line is metal, anything right is non-metal, like this...

2.1 understand the terms group and period
Groups
A group is a column in the periodic table. All elements in a group have similar chemical properties (because they have the same number of electrons on their outer shell), however, the properties of each element (such as reactivity) often gradually change as you go down a group (as the atomic number increases).
Periods
A period is a row in the periodic table. Properties of elements in the same period are not similar and quite often change.
A group is a column in the periodic table. All elements in a group have similar chemical properties (because they have the same number of electrons on their outer shell), however, the properties of each element (such as reactivity) often gradually change as you go down a group (as the atomic number increases).
Periods
A period is a row in the periodic table. Properties of elements in the same period are not similar and quite often change.
Tuesday, 22 March 2016
1.54 describe experiments to investigate electrolysis, using inert electrodes, of aqueous solutions such as sodium chloride, copper(II) sulphate and dilute sulphuric acid and predict the products
In aqueous solutions, as well as ions from the ionic compound, there will be hydrogen ions (H+) and hydroxide ions (OH-) from the water.
Products
At the cathode, if H+ ions and metal ions are present, hydrogen gas will be produced if the metal ions are more reactive than H+ ions (for example, sodium ions). If the metal ions are less reactive than the H+ ions (for example, copper ions), a solid layer of the pure metal will be produced.
At the anode, if OH- and halide ions (Cl-, Br-, I-) are present, then molecules of chlorine, bromine or iodine will be formed. If no halide ions are present, then oxygen gas and water will be formed.
Electrolysis of sulphuric acid
A solution of sulphuric acid (H2SO4) contains three different ions: SO42− , H+ and OH-.
At the cathode: as sulphur (SO42−) is more reactive than hydrogen, hydrogen gas is produced...
2H+ + 2e- ---> H2
At the anode: as there no halide ions present, oxygen and water is produced.
4OH- ---> O2 + 2H2O + 4e-
Electrolysis of sodium chloride
A solution of sodium chloride (NaCl) contains four different ions: Na+, Cl-, OH- and H+
At the cathode: as sodium is more reactive than hydrogen, hydrogen gas is produced...
2H+ + 2e- ---> H2
At the anode: as chlorine ions are present (halide), then chlorine atoms will be produced (as chlorine gas)...
2Cl- ---> Cl2 + 2e-
Electrolysis of copper(II) sulfate
A solution of copper(II) sulphate (CuSO4) contains four different ions: Cu2+, SO42−, H+ and OH-.
At the cathode: as copper is less reactive than hydrogen, copper metal is produced...
Cu2+ + 2e- ---> Cu
At the anode: as there are no halide ions present, oxygen and water are produced...
4OH- ---> O2 + 2H2O + 4e-
Products
At the cathode, if H+ ions and metal ions are present, hydrogen gas will be produced if the metal ions are more reactive than H+ ions (for example, sodium ions). If the metal ions are less reactive than the H+ ions (for example, copper ions), a solid layer of the pure metal will be produced.
At the anode, if OH- and halide ions (Cl-, Br-, I-) are present, then molecules of chlorine, bromine or iodine will be formed. If no halide ions are present, then oxygen gas and water will be formed.
Electrolysis of sulphuric acid
A solution of sulphuric acid (H2SO4) contains three different ions: SO42− , H+ and OH-.
At the cathode: as sulphur (SO42−) is more reactive than hydrogen, hydrogen gas is produced...
2H+ + 2e- ---> H2
At the anode: as there no halide ions present, oxygen and water is produced.
4OH- ---> O2 + 2H2O + 4e-
Electrolysis of sodium chloride
A solution of sodium chloride (NaCl) contains four different ions: Na+, Cl-, OH- and H+
At the cathode: as sodium is more reactive than hydrogen, hydrogen gas is produced...
2H+ + 2e- ---> H2
At the anode: as chlorine ions are present (halide), then chlorine atoms will be produced (as chlorine gas)...
2Cl- ---> Cl2 + 2e-
Electrolysis of copper(II) sulfate
A solution of copper(II) sulphate (CuSO4) contains four different ions: Cu2+, SO42−, H+ and OH-.
At the cathode: as copper is less reactive than hydrogen, copper metal is produced...
Cu2+ + 2e- ---> Cu
At the anode: as there are no halide ions present, oxygen and water are produced...
4OH- ---> O2 + 2H2O + 4e-
1.53 describe experiments to investigate electrolysis, using inert electrodes, of molten salts such as lead(II) bromide and predict the products.
Firstly, inert electrodes are just ones that don't react easily (like at all).
NOTE: The cathode (negative) attracts Pb2+ ions as they are positive, the anode (positive) attracts Br- ions as they are negative
 |
NOTE: The cathode (negative) attracts Pb2+ ions as they are positive, the anode (positive) attracts Br- ions as they are negative
- As soon as the lead(II) Bromide melts(becomes molten), the ions become free to move around, this movement enables the ions move allowing a charge to flow, meaning electrolysis can take place.
- The electrodes are made out of carbon - which is inert (unreactive).
- Connect the electrodes to a power source
- The positive lead (II) ions are attracted to the cathode, which is the negative electrode. When they get there, they gain 2 electrons each from the electrode. This forms lead atoms (they are no longer ions as they have no charge). These 'fall' to the bottom of the container as molten lead.
- Bromide ions (negative) are attracted to the positive anode. When they get there, the extra electron which makes the bromide ion negatively charged moves onto the anode, this loss of the extra electron turns each bromide ion into a bromine atom. These join in pairs (bond covalently) to form bromine molecules (which is gas).
The half equations...
At the cathode: Pb2+ + 2e- ----> Pb
At the anode: 2Br- ---> Br2 + 2e-
1.52 understand that electrolysis involves the formation of new substances when ionic compounds conduct electricity
During electrolysis, ionic compounds conduct electricity (because they are molten so they have free ions). During electrolysis, negative ions (anions) are attracted to the positive electrode (the anode) and positive ions (cations) are attracted to the negative electrode (the cathode). For the circuit to be complete there has to be a flow of electrons. For this to occur, electrons are taken away from negative ions at the anode (positive electrode) and given to positive ions at the cathode (negative electrode). This means that the ions in the electrolyte undergo a reaction whereby they either gain or lose electrons (reduction or oxidation) and so a new product is formed at each electrode (because as ions lose electrons, they become atoms or molecules).
1.51 describe experiments to distinguish between electrolytes and non-electrolytes
When you place a conductivity probe in an electrolyte, current will flow through the circuit - this means you can measure its conductivity.
When you place conductivity probe in a non-electrolyte, no current will flow - this means you will get a reading of 0 conductivity
Another way to determine whether a substance is an electrolyte or non-electrolyte is to set up an electrolytic cell - if the substance will undergo electrolysis then it is an electrolyte, if not, it is not.
When you place conductivity probe in a non-electrolyte, no current will flow - this means you will get a reading of 0 conductivity
Another way to determine whether a substance is an electrolyte or non-electrolyte is to set up an electrolytic cell - if the substance will undergo electrolysis then it is an electrolyte, if not, it is not.
1.50 understand why ionic compound conduct electricity only when molten or in solution
Electrolysis requires an electrolyte (a liquid that an conduct electricity), electrolytes are made by melting or dissolving ionic compounds, this results in free ions which conduct electricity. Molten ionic compounds can conduct electricity because the ions can move freely.
1.49 understand why covalent compounds do not conduct electricity
In a covalent compound, there are no delocalised electrons, so it cannot hold a current (in other words, there are no electrons free to move, therefore there can be no transfer of electricity).
NOTE: Graphite is an exception as this is a giant covalent structure and has the 4th ion free to move, so graphite can conduct electricity
1.48 understand that an electric current is a flow of electrons or ions
An electric current is a flow of electrons, it an also be a flow of ions (as the are charged). Its basically a flow of charged particles.
1.47 explain the electrical conductivity and malleability of a metal in terms of its structure and bonding.
Metals are a giant 3-D structure of positive ions surrounded by delocalized electrons.
Electrons carry electricity. Metals are good conductors of electricity as they have lots of delocalized ('free') electrons that are free to move when a voltage is applied, carrying a charge through the metal.
Metals are structured with layers of positive ions on top of eachother. These ions can easily slide over one another as (in pure metals) they will all be the same size. As they can easily slide, this means they are malleable and ductile.
NOTE: The metallic bonds (force between cations and delocalized electrons) are not broken when metals are moulded as the electrons 'travel' with the cations.
Electrons carry electricity. Metals are good conductors of electricity as they have lots of delocalized ('free') electrons that are free to move when a voltage is applied, carrying a charge through the metal.
Metals are structured with layers of positive ions on top of eachother. These ions can easily slide over one another as (in pure metals) they will all be the same size. As they can easily slide, this means they are malleable and ductile.
NOTE: The metallic bonds (force between cations and delocalized electrons) are not broken when metals are moulded as the electrons 'travel' with the cations.
1.45 explain how the uses of diamond and graphite depend on their structures, limited to graphite as a lubricant and diamond in cutting
Diamond
In diamond, each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. As a result, diamond is very hard and has a high melting point. This explains why it is used in cutting tools
Graphite
In graphite, each carbon atom is joined to only three other carbon atoms, this results in carbon sheets that are 'stacked' on top of each other. These layers can slide over each other, this means that graphite is much softer than diamond. It is used in pencils, and as a lubricant
NOTE: Diamond does not conduct electricity but graphite does
In diamond, each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. As a result, diamond is very hard and has a high melting point. This explains why it is used in cutting tools
Graphite
In graphite, each carbon atom is joined to only three other carbon atoms, this results in carbon sheets that are 'stacked' on top of each other. These layers can slide over each other, this means that graphite is much softer than diamond. It is used in pencils, and as a lubricant
NOTE: Diamond does not conduct electricity but graphite does
1.43 explain the high melting and boiling points of substances with giant covalent structures in terms of the breaking of many strong covalent bonds
Giant covalent structures are very like giant ionic structures only they do not have charged ions. Instead, all atoms are bonded to each other by strong covalent bonds. There are a lot of these bonds which means it takes a lot of energy to break them, therefore they have very high melting and boiling points
1.42 explain why substances with simple molecular structure have low melting and boiling points in terms of the relatively weak forces between the molecules
The atoms within a molecule are held together with strong covalent bonds. In contrast, the intermolecular forces between molecules are very weak and as a result the boiling and melting points of simple molecular substances are very low (because the molecules are easily parted from each other)
1.41 understand that substances with simple molecular structures are gases or liquids, or solids wit low melting points
You can tell when a substance has a simple molecular structure from its physical state (at room temperature). Most molecular substances are liquid or gas (at room temperature) but some are solid with a low melting point
1.40 explain, using dot and cross diagrams, the formation of covalent compounds by electron sharing for the following substances...
For each of the diagrams I have added a little explanation of what's happening to aid understanding, if you know what happens ignore the writing, otherwise read it as it may be useful :)
Hydrogen (H2)
Hydrogen atoms only have 1 electron and they only need 1 more to compete their shell (remember: the first shell only needs two electrons to be complete). To complete their shell, they form a covalent bond. Only one pair of electrons is shared between them, this molecule is known as H2 (hydrogen gas)

Chlorine (Cl2)
Much like hydrogen, chlorine atoms also only need one more electron...

This is Cl2 (as there are 2 Cl atoms in the molecule)
Hydrogen chloride (HCl)
As hydrogen and chlorine both only need one electron to complete their outer shell, they can bond with each other, this forms HCl...

Water (H2O)
Oxygen atoms have 6 electrons on their outer shell and therefore need 2 more electrons to complete their shell. However, hydrogen only needs one. This means that one oxygen atom must bond with two hydrogen atoms...

Methane (CH4)
Carbon has 4 outer electrons, therefore it needs 4 more to complete its outer electron shell. However, hydrogen only needs 1 more electron. This means that one carbon atom bonds with four hydrogen atoms

Ammonia (NH3)
Nitrogen has 5 electrons on its outer shell, so needs 3 more electrons to compete its shell, but hydrogen only needs 1 more electron. This means that 1 nitrogen atom bonds with 3 hydrogen atoms...

Oxygen (O2)
In oxygen gas one oxygen atom shares TWO pairs of electrons with another oxygen atom to complete its shell, this is known as a double covalent bond

Nitrogen (N2)
Nitrogen atoms have 5 electrons on their outer shell, therefore they need 3 more electrons to complete their shell. This means that two nitrogen atoms share THREE pairs of electrons to fill their outer shells, this is known as a triple covalent bond

Carbon dioxide (CO2)
In carbon dioxide. two oxygen atoms share two pairs of electrons with a carbon atom, this forms two double covalent bonds
Ethane (C2H6)
In ethane there are two carbon atoms and six hydrogen atoms. Each of the six hydrogen atoms share their only electron with one of the two carbon atoms (each carbon atom bonds with 3 hydrogen atoms), the two carbon atoms then share their last electron with each other.
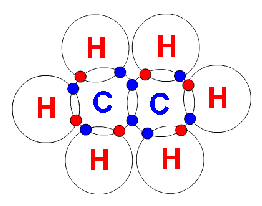
Ethene (C2H4)
In there there are two carbon atoms and four hydrogen atoms. The four hydrogen atoms each share their only electron with one of two carbon atoms, the two carbon atoms then share their lat two electrons with each other, forming a carbon-carbon double covalent bond.
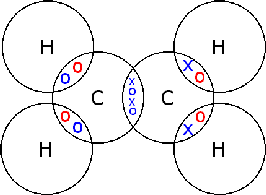
Hydrogen (H2)
Hydrogen atoms only have 1 electron and they only need 1 more to compete their shell (remember: the first shell only needs two electrons to be complete). To complete their shell, they form a covalent bond. Only one pair of electrons is shared between them, this molecule is known as H2 (hydrogen gas)

Chlorine (Cl2)
Much like hydrogen, chlorine atoms also only need one more electron...

This is Cl2 (as there are 2 Cl atoms in the molecule)
Hydrogen chloride (HCl)
As hydrogen and chlorine both only need one electron to complete their outer shell, they can bond with each other, this forms HCl...

Water (H2O)
Oxygen atoms have 6 electrons on their outer shell and therefore need 2 more electrons to complete their shell. However, hydrogen only needs one. This means that one oxygen atom must bond with two hydrogen atoms...

Methane (CH4)
Carbon has 4 outer electrons, therefore it needs 4 more to complete its outer electron shell. However, hydrogen only needs 1 more electron. This means that one carbon atom bonds with four hydrogen atoms

Ammonia (NH3)
Nitrogen has 5 electrons on its outer shell, so needs 3 more electrons to compete its shell, but hydrogen only needs 1 more electron. This means that 1 nitrogen atom bonds with 3 hydrogen atoms...

Oxygen (O2)
In oxygen gas one oxygen atom shares TWO pairs of electrons with another oxygen atom to complete its shell, this is known as a double covalent bond

Nitrogen (N2)
Nitrogen atoms have 5 electrons on their outer shell, therefore they need 3 more electrons to complete their shell. This means that two nitrogen atoms share THREE pairs of electrons to fill their outer shells, this is known as a triple covalent bond

Carbon dioxide (CO2)
In carbon dioxide. two oxygen atoms share two pairs of electrons with a carbon atom, this forms two double covalent bonds
Ethane (C2H6)
In ethane there are two carbon atoms and six hydrogen atoms. Each of the six hydrogen atoms share their only electron with one of the two carbon atoms (each carbon atom bonds with 3 hydrogen atoms), the two carbon atoms then share their last electron with each other.

Ethene (C2H4)
In there there are two carbon atoms and four hydrogen atoms. The four hydrogen atoms each share their only electron with one of two carbon atoms, the two carbon atoms then share their lat two electrons with each other, forming a carbon-carbon double covalent bond.

1.39 understand covalent bonding as a strong attraction between the bonding pair of electrons and the nuclei of the atoms involved in the bond
sorry not much to say here... you just need to learn that in covalent bonding there is a strong attraction between the shared electrons (the 'bonding pair') and the nuclei of the atoms involved
1.38 describe the formation of a covalent bond by sharing a pair of electrons between the two atoms
Covalent bonding is where atoms share electrons with each other so that they have a full outer shell (as aposed to ionic bonding where an electron is 'given away' to another atom)
1.37 draw a diagram to represent the positions of the ions in a crystal of sodium chloride
Sodium chloride has a typical ionic structure, it has alternating positive (Na+) and negative (Cl-) ions, this is how it should be drawn...


1.36 describe an ionic crystal as a giant three-dimensional lattice structure held together by the attraction between oppositely charged ions
Compounds with ionic bonding always have giant ionic structures, the ions are held closely together by the attraction between oppositely charged ions in a 3-D lattice arrangement.
Sunday, 20 March 2016
1.35 understand the relationship between ionic charge and the melting point and boiling point of an ionic compound
Basically, the bigger the charge (e.g 3-), the stronger the electrostatic forces. This means that the bigger the ionic charge, the higher the melting/boiling point of the ion(s)
1.34 understand that ionic compounds have high melting and boiling points because of strong electrostatic forces between oppositely charged ions
Ionic compounds are held together by strong electrostatic forces that hold them together due to the attraction of the opposing charges (+ and -). To melt or boil anything, heat is used to break the electrostatic forces. The force will get stronger the higher the charge of the ion is, therefore, the stronger the bonds, the more heat needed. Ionic compounds have strong bonds, so they don't melt or boil unless there is a considerable amount of heat, this means they have high melting and boiling points.
1.33 understand ionic bonding as strong electrostatic attraction between oppositely charged ions
In ionic bonding, atoms gain or lose electrons to form charged particles (ions) which are then strongly attracted to each other (because of the attraction of opposite charges, + and -). This strong attraction is known as electrostatic attraction. This electrostatic attraction gives ionic compounds high melting and boiling points.
1.32 explain, using dot and cross diagrams, the formation of ionic compounds by electron transfer, limited to combinations of elements rom groups 1, 2, 3 and 5, 6, 7
Dot and cross diagrams show what's happening regarding the electrons when ionic bonding happens, the best way to explain is just to show you
Sodium Chloride (NaCl)
Sodium ions have the formula Na+ and chloride ions have the formula Cl-. To have an outer shell, sodium wants to lose an electron and chloride wants to gain an electron. To make this happen, sodium gives chloride an electron. In the exam, make sure the dots and crosses are clear.



Sodium Chloride (NaCl)
Sodium ions have the formula Na+ and chloride ions have the formula Cl-. To have an outer shell, sodium wants to lose an electron and chloride wants to gain an electron. To make this happen, sodium gives chloride an electron. In the exam, make sure the dots and crosses are clear.

Magnesium oxide (MgO)

Magnesium ions have the formula Mg2+, while oxide ions have the formula O2-. Magnesium gives oxygen two electrons
Calcium chloride, CaCl2 (a little trickier)

Calcium ions have the formula Ca2+. Chloride ions have the formula Cl-. Calcium wants to lose 2 electrons but chloride only wants 1. Therefore, two chloride ions bond with one calcium ion, this means that each chloride ion gets 1 electron and calcium loses two, so everyone happy :)
Image source: bitesize
Image source: bitesize
1.30 recall the charges of common ions in this specification
All this means is that we've got to learn a few charges...
CATIONS
Group 1
Na+
Li+
K+
Group 2
Ca2+
Be2+
Mg2+
ANIONS
Group 6
O2-
S2-
Group 7 (halogens)
F-
Cl-
Br-
CATIONS
Group 1
Na+
Li+
K+
Group 2
Ca2+
Be2+
Mg2+
ANIONS
Group 6
O2-
S2-
Group 7 (halogens)
F-
Cl-
Br-
1.29 understand oxidation as the loss of electrons and reduction as the gain of electrons
In ionic bonding, atoms either lose or gain electrons (to form ions). When an atom loses electrons, it is called oxidation, when an atom gains electrons, it is called reduction.
NOTE: a useful was of remembering which is which is with the anagram 'O.I.L.R.I.G' (Oxidation.Is.Loss.Reduction.Is.Gain)
NOTE: a useful was of remembering which is which is with the anagram 'O.I.L.R.I.G' (Oxidation.Is.Loss.Reduction.Is.Gain)
1.28 describe the formation of ions by the gain or loss of electrons
An ion is any atom (or group of atoms) that has charge / is electrically charged. Ions are formed due to the loss or gain of electrons.
If an atom gains an electron it becomes a negatively charged ion. Non-metals tend to do this, and they form anions. So elements from group 5-7 will form anions.
If an atom loses an electron it becomes a positively charged ion.the ion has a positive charge. Metals tend to do this, and they form cations (positive ions), so normally elements from group 1-3 will form cations.
Group 0/8 are the noble gases and are inert + unreactive, so they do not form ions.
NOTE: an easy way to remember anion is A-Negative-ION - ANIONAn easy way to remember a cation is that if its not an anion, its a cation :)
If an atom gains an electron it becomes a negatively charged ion. Non-metals tend to do this, and they form anions. So elements from group 5-7 will form anions.
If an atom loses an electron it becomes a positively charged ion.the ion has a positive charge. Metals tend to do this, and they form cations (positive ions), so normally elements from group 1-3 will form cations.
Group 0/8 are the noble gases and are inert + unreactive, so they do not form ions.
NOTE: an easy way to remember anion is A-Negative-ION - ANIONAn easy way to remember a cation is that if its not an anion, its a cation :)
Saturday, 19 March 2016
1.27 carry out mole calculations using volumes and molar concentrations
For this we need to use a few equations (it is best to show with an example)...
Calculate the volume of carbon dioxide produced when 50g of calcium carbonate is decomposed by heating.
Step 1 - Calculate the amount, in moles, of calcium carbonate reacted
work out the Mr of CaCO... it's 100
Therefore, we can work out the amount, in moles, of calcium carbonate that was reacted...
Amount, in moles, of CaCO₃ = (50 ÷ 100) = 0.5 mol
Step 2 - Calculate the amount, in moles, of carbon dioxide formed
CaCO₃ ------> CaO + CO₂
1 mol of CaCO₃ produces 1 mol of CO₂, therefore, 0.5 mol of CaCO₃ must produce 0.5 mol of CO₂
Step 3 - Calculate the volume of CO₂ formed
Volume of CO₂ (in dm3) = (0.5 x 24) dm³ = 12dm³
So, the volume of carbon dioxide produced when 50g of calcium carbonate is decomposed by heating, is 12dm3
Calculate the volume of carbon dioxide produced when 50g of calcium carbonate is decomposed by heating.
Step 1 - Calculate the amount, in moles, of calcium carbonate reacted
work out the Mr of CaCO... it's 100
Therefore, we can work out the amount, in moles, of calcium carbonate that was reacted...
Amount, in moles, of CaCO₃ = (50 ÷ 100) = 0.5 mol
Step 2 - Calculate the amount, in moles, of carbon dioxide formed
CaCO₃ ------> CaO + CO₂
1 mol of CaCO₃ produces 1 mol of CO₂, therefore, 0.5 mol of CaCO₃ must produce 0.5 mol of CO₂
Step 3 - Calculate the volume of CO₂ formed
Volume of CO₂ (in dm3) = (0.5 x 24) dm³ = 12dm³
So, the volume of carbon dioxide produced when 50g of calcium carbonate is decomposed by heating, is 12dm3
1.26 calculate percentage yield
Percentage yield is calculated with the equation...
Percentage yield = (actual yield (grams) / theoretical yield (grams)) x 100
NOTE: a 100% yield means you got all the product you expected to get; a 0% yield means that no reactants were converted into product
Percentage yield = (actual yield (grams) / theoretical yield (grams)) x 100
NOTE: a 100% yield means you got all the product you expected to get; a 0% yield means that no reactants were converted into product
1.25 calculate reacting masses using experimental data and chemical equations
1 - write out the balanced equation (you may be given this in an exam)
2 - for the two bits you want, work out the relative formula mass (Mr) and multiply them by the balancing numbers in the equation
3 - Apply the rule : divide to get one, them multiply to get all (but you have to apply the first to the substance they give information about, and then the other one)
For example: What mass of magnesium oxide is produced when 60g of magnesium is burnt is air?
Step 1: balanced equation...
2Mg + O2 -----> 2MgO
Step 2: work out the relative formula masses of each component and multiply them by the balancing numbers in the equation (sounds confusing, sorry)
2Mg: 2 x 24 = 48 2MgO: 2 x (24 x 16) = 80
Step 3: apply the rule 'divide to get one, multiply to get all'...
The two numbers, 48 and 80, tell us that 48g of Mg react to give 80g of MgO. However, the question asks us how much MgO is produced when 60g of Mg is reacted, so we need to work out how much MgO is produced when 60g of Mg is reacted...
48g of Mg........80g of MgO (divide by the reactant, in this case, divide by 48)
1g of Mg..........1.67g of MgO ( x by 60, as thats how much Mg we have)
60g of Mg........100g of MgO
Therefore, 60g of Mg reacts with air to produce 100g of MgO
2 - for the two bits you want, work out the relative formula mass (Mr) and multiply them by the balancing numbers in the equation
3 - Apply the rule : divide to get one, them multiply to get all (but you have to apply the first to the substance they give information about, and then the other one)
For example: What mass of magnesium oxide is produced when 60g of magnesium is burnt is air?
Step 1: balanced equation...
2Mg + O2 -----> 2MgO
Step 2: work out the relative formula masses of each component and multiply them by the balancing numbers in the equation (sounds confusing, sorry)
2Mg: 2 x 24 = 48 2MgO: 2 x (24 x 16) = 80
Step 3: apply the rule 'divide to get one, multiply to get all'...
The two numbers, 48 and 80, tell us that 48g of Mg react to give 80g of MgO. However, the question asks us how much MgO is produced when 60g of Mg is reacted, so we need to work out how much MgO is produced when 60g of Mg is reacted...
48g of Mg........80g of MgO (divide by the reactant, in this case, divide by 48)
1g of Mg..........1.67g of MgO ( x by 60, as thats how much Mg we have)
60g of Mg........100g of MgO
Therefore, 60g of Mg reacts with air to produce 100g of MgO
1.24 calculate empirical and moleculer formulae from experimental data
Empirical (and molecular) formula can also be calculated from experimental data. This is how its done...
- List all the elements in the compound
- Underneath, write their experimental masses or percentages
- Divide each mass by the Ar (relative atomic mass) of that particular element
- Turn the numbers into a ratio
- Simplify the ratio
For example: In an experiment, some iron oxide powder is reduced to pure metallic iron. Use the following experimental data to find the empirical formula of the iron oxide used.
Mass of empty container = 32.0g
Mass of container + mass of iron oxide = 96.0g
Mass of container + iron = 76.8g
METHOD -
During this experiment, oxygen is lost. To find the mass of oxygen lost, minus the 'mass of container + iron' from 'mass of container + mass of iron oxide'...
96 - 76.8 = 19.2g
The mass of iron made is 'mass of container + iron' minus 'mass of container'...
76.8 - 32 = 44.8g
Now, list the elements in iron oxide...
Fe O
write their experimental masses
44.8 19.2
divide by their Ar
44.8 / 56 = 0.8 19.2 / 16 = 1.2
multiply by 10 to put into ratio
8 : 12
cancel down
2 : 3
This means that the simplest formula is 2 Fe atoms to every 3 O atoms, so the empirical formula is Fe2O3
- List all the elements in the compound
- Underneath, write their experimental masses or percentages
- Divide each mass by the Ar (relative atomic mass) of that particular element
- Turn the numbers into a ratio
- Simplify the ratio
For example: In an experiment, some iron oxide powder is reduced to pure metallic iron. Use the following experimental data to find the empirical formula of the iron oxide used.
Mass of empty container = 32.0g
Mass of container + mass of iron oxide = 96.0g
Mass of container + iron = 76.8g
METHOD -
During this experiment, oxygen is lost. To find the mass of oxygen lost, minus the 'mass of container + iron' from 'mass of container + mass of iron oxide'...
96 - 76.8 = 19.2g
The mass of iron made is 'mass of container + iron' minus 'mass of container'...
76.8 - 32 = 44.8g
Now, list the elements in iron oxide...
Fe O
write their experimental masses
44.8 19.2
divide by their Ar
44.8 / 56 = 0.8 19.2 / 16 = 1.2
multiply by 10 to put into ratio
8 : 12
cancel down
2 : 3
This means that the simplest formula is 2 Fe atoms to every 3 O atoms, so the empirical formula is Fe2O3
Subscribe to:
Comments (Atom)


